2023 年 92 巻 2 号 p. 105-114
2023 年 92 巻 2 号 p. 105-114
A major challenge in terms of commercializing 1-methylclopropene (1-MCP) to extend the storage life and control physiological disorders in European pears is that it irreversibly inhibits fruit ripening in some cultivars, particularly flesh softening that is necessary for optimal consumption quality. In this study, we examined the effect of 1-MCP pretreatments on fruit ripening and associated transcriptomic changes in ‘La France’ (Pyrus communis L.) pears during storage at 20°C and 5°C. Compared to non-treated controls, 1-MCP pretreatment suppressed fruit respiration and ethylene production rates, and markedly delayed flesh softening. Normal ripening (ethylene production and flesh softening to eating quality firmness) was observed in 1-MCP treated fruit after 42 d at 20°C, and 112 d at 5°C. Subsequent RNA-Seq analysis revealed that 6,427 genes, including those associated with ethylene biosynthesis (ACS1, ACS1b, ACO1, and ACO2), cell wall degrading enzymes (PG3, β-GAL, EG, and EXP1), and transcription factors (AGL18 and NAC29) were up- or down-regulated in non-treated fruit both at 20°C and 5°C. The expression patterns of these genes were disrupted by 1-MCP pretreatment, but up- or down-regulation was also observed when ethylene was detected in 1-MCP-treated fruit. Together, these findings demonstrate the potential for practical use of 1-MCP to extend storage life in ‘La France’ pears given that (i) a single application markedly extended storage life to 56 d at 20°C and 112 d at 5°C, and (ii) treated fruit could regain their softening capacity, thus eliminating previous irreversible ripening blockage concerns.
In most European pear cultivars, flesh softening is an integral part of fruit ripening, and the resultant buttery texture is an important criterion for optimal consumption quality (Nicolaï et al., 2008). Flesh softening arises largely from major alterations in the structure and composition of primary cell walls, which result in loss of intercellular wall adhesion, wall loosening and disintegration (Wang et al., 2019). Such alterations are complex and involve the coordinated and interdependent action of various enzyme and protein families (Guo et al., 2018; Wang et al., 2019; Xiao et al., 2019), including polygalacturonase (PG), endo-1,4-β-glucanase (EG), β-galactosidase (β-GAL), and expansin (EXP) among others. Genes encoding cell wall degrading enzymes and proteins during fruit ripening-associated flesh softening have been identified and well characterized (Bennett and Labavitch, 2008).
European pears are classified as climacteric fruit, and hence, fruit ripening-associated flesh softening is typically regulated by ethylene (Hiwasa et al., 2003). The underlying molecular mechanisms of ethylene-regulated fruit ripening have been studied, and it is proposed that several transcription factors (TFs) trigger signaling pathways that control ethylene biosynthesis, fruit softening, and other ripening-related events (Liu et al., 2015). However, unlike other climacteric fruit, most European pear cultivars require low temperature treatments to produce ethylene and ripen properly (Villalobos-Acuña and Mitcham, 2008). Low temperature treatments have been shown to stimulate the activities of ethylene biosynthetic enzymes 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) and ACC oxidase (ACO), as well as the expression levels of corresponding genes (El-Sharkawy et al., 2004; Fonseca et al., 2005). Furthermore, the expression levels of various genes encoding cell wall degrading enzymes and proteins have been shown to rapidly increase after low temperature treatments (Fonseca et al., 2005; Mitalo et al., 2019a).
Despite its contribution to edibility and palatability, flesh softening renders fruit susceptible to physical damage and postharvest pathogen attacks that may limit fruit transportation and storage practices (Meli et al., 2010; Yang et al., 2017). Several strategies have thus been employed to extend the postharvest life of European pears. Among them, cold storage is the most widely used, but prolonged storage at the recommended temperatures (usually −1.1°C) causes physiological disorders like superficial scalds, brown heart and core breakdown (Dias et al., 2021). Furthermore, controlled and modified systems can also extremely prolong postharvest life (Drake et al., 2004), but the resultant accumulation of CO2 increases the incidence of internal browning and thus lower fruit quality (Wang and Sugar, 2013).
1-Methylcyclopropene (1-MCP) is a potent ethylene action inhibitor (Sisler and Serek, 2003) that has been shown to delay fruit ripening and extend postharvest life in many climacteric fruit (Watkins, 2006). In European pears, it has been broadly demonstrated that postharvest application of 1-MCP reduces ethylene production and fruit softening, while also preventing development of superficial scald, internal browning, and other physiological disorders (Chiriboga et al., 2011; Ekman et al., 2004; Xie et al., 2014). However, the major downside of postharvest 1-MCP application is that it causes irreversible inhibition of fruit ripening in several cultivars including ‘Bartlett’ (Villalobos-Acuña and Mitcham, 2008), ‘d’Anjou’ (Argenta et al., 2003), ‘Conference’ (Chiriboga et al., 2011), and ‘Blanquilla’ (Chiriboga et al., 2010). Consequently, practical usage of 1-MCP to extend storage life in other European pear cultivars such as ‘La France’ for which there are no known reports of ripening blockage, has been limited. Furthermore, efforts to avoid or reverse 1-MCP-induced fruit ripening blockage including heat treatments (Chiriboga et al., 2010), post-storage ethylene treatments (Villalobos-Acuña et al., 2011; Xie et al., 2014), and glycoxylic acid treatment (Hewitt et al., 2020) are either costly or have yielded conflicting results. With these considerations in mind, postharvest strategies that could circumvent post-storage treatments to reverse 1-MCP-induced fruit ripening blockage are highly desirable.
In the present study, we examined the effect of a single 1-MCP application prior to storage at low temperature (5°C) or room temperature (20°C) on ethylene production, respiration, and flesh softening rates of ‘La France’ European pears. Using RNA-Seq to analyze the transcriptome, we demonstrate that 1-MCP-treated fruit can indeed regain their softening capacity after a certain period that is dependent on storage temperature, thus potentially negating the need for post-storage treatments.
‘La France’ pear (Pyrus communis L.) fruit at a pre-climacteric stage were obtained from a commercial orchard in Yamagata, Japan. Careful sorting was then carried out to ensure uniform color, size, and shape, and to select fruit that were free from mechanical injury, insect damage and diseases. The fruit were then divided into two major groups of 80 fruit each. The first group was treated with 1-MCP (2 μL·L−1) for 12 h at 20°C. 1-MCP was released by dissolving SmartFreshTM powder (AgroFresh, PA, USA) in water as previously described (Mitalo et al., 2019a). The second group was kept in air for 12 h at 20°C as a non-treated control. After treatment, both the control and 1-MCP treated groups were further divided into two groups of forty fruit each for storage trials at either 5°C or 20°C. During storage, each fruit was individually wrapped in perforated polyethene bags to allow gaseous exchange and to prevent water loss.
Determination of fruit ripening and quality characteristicsRespiration and ethylene production rates were determined as described previously (Hira et al., 2022), using 10 independent replications (10 fruit). Individual fruit were incubated at the respective storage temperatures in a 1360-mL airtight container for up to 1 h. To determine the ethylene production rate, 2 mL of headspace gas was withdrawn and injected into a gas chromatograph (Model-GC4 CMPF, Shimadzu, Kyoto, Japan) equipped with a flame ionization detector (set at 200°C) and an activated alumina column (set at 80°C) (Mitalo et al., 2019a). The respiration rate was determined by injecting 2 mL of headspace gas into a gas chromatograph (model-GC4 CMPF, Shimadzu) equipped with a thermal conductivity detector and a Porapak Q column. Values were expressed in terms of CO2 production rate.
Flesh firmness was measured at four different equatorial regions of the fruit, after the skin had been removed, using a rheometer (model Rheutech, Fudoh, Tokyo, Japan) with a maximum force of 10 kg and a 3-mm diameter round stainless-steel probe with a flat end (Hiwasa et al., 2003). Data were recorded as Newtons (N) and values were expressed as means of three independent biological replications (three fruit).
Soluble solids content (SSC) of the fruit juice was measured using a digital Atago PR1 refractometer (Atago Co. Ltd., Tokyo, Japan), and the values were expressed as a percentage. Titratable acidity (TA) was determined by titrating fruit juice against 0.1 N NaOH using phenolphthalein as a pH indicator, and values were expressed as percentage citric acid equivalents.
After the above assessments, flesh tissue samples were collected, cut into small pieces and frozen in liquid nitrogen before storage at −80°C for future analysis.
Transcriptome library construction and sequencingSamples used for this analysis were collected at harvest: (0 d), 20°C control (14 d), 20°C 1-MCP pretreated (14, 42 and 56 d), 5°C control (28 and 56 d), and 5°C 1-MCP pretreated (28, 56, and 84 d). Total RNA was extracted from 1.5 g ground flesh tissue of three replicate fruits using the hot borate method (Wan and Wilkins, 1994). Treatment with DNase I (Nippon Gene, Tokyo, Japan) was used to remove genomic DNA contamination, and further purification was carried out using FavorPrep after Tri-Reagent RNA Clean-up Kit (Favorgen Biotech Co., Ping-Tung, Taiwan). The resulting clean RNA was used to construct Illumina paired-end libraries using a KAPA RNA HyperPrep Kit for Illumina® (KAPA Biosystems) (New England Biolabs, MA, USA). Sequencing was carried out using an Illumina HiSeq platform (Macrogen Co. Ltd., Japan) and sequenced reads were trimmed to exclude low-quality nucleotide sequences (Phred score < 20) and to remove adapter sequences, as previously reported (Mitalo et al., 2020).
Differential gene expression analysisTrimmed Illumina reads were mapped to the reference Pyrus communis Bartlett DH Genome v2.0 (Linsmith et al., 2019), and the mapped reads were counted using a CLC genomic workbench (CLC Bio-Qiagen, Aarhus, Denmark) with default settings. Gene expression levels were normalized as reads per kilobase million (RPKM). Differentially expressed genes (DEGs) were detected through comparisons between fruit samples obtained at harvest (0 d) and those 1-MCP pretreated and non-treated (control) with the edgeR package. Selection of DEGs was based on three criteria: (i) RPKM ≥ 1.0, (ii) three-fold increase or decrease in expression, and (iii) false discovery rate ≤ 0.01. Clustering and heatmap analysis of the DEGs were performed using the hclst command and the heatmap package, respectively, in R.
Statistical analysisData obtained in this study were analyzed using R software (version 4.0.0, R project). ANOVA followed by post hoc Tukey’s tests (P < 0.05) were used to detect differences in respiration, ethylene production, flesh firmness, and gene expression among the different treatments.
During storage at 20°C, non-treated control fruit displayed a rapid increase in respiration rates (Fig. 1A), which peaked at 14 d (CO2 ~320 nmol·kg−1·s−1). Ethylene production rates of control fruit also increased rapidly (Fig. 1C), peaking at 21 d (~26 nmol·kg−1·s−1), and flesh firmness values dramatically decreased to eating quality (2 N) at 14 d (Fig. 1E). On the other hand, fruit that were pretreated with 1-MCP displayed few changes in either respiration or ethylene production rates for up to 42 d; substantial increases were recorded later at 56 d. Flesh firmness of 1-MCP-pretreated fruit remained relatively unchanged for 42 d, but a rapid decrease was observed at 56 d in accordance with increased ethylene and respiration rates. Even then, 1-MCP pretreated fruit at 56 d were twice as firm (~4 N) than control fruit at 14 d (~2 N). Changes in SSC were negligible, while TA values slightly decreased both in 1-MCP pretreated and control fruit (Supplementary Fig. 1A, C). These findings indicate that a single 1-MCP application prior to storage at 20°C halted fruit ripening in ‘La France’ pears for at least 42 d, while ripening recovery after this.
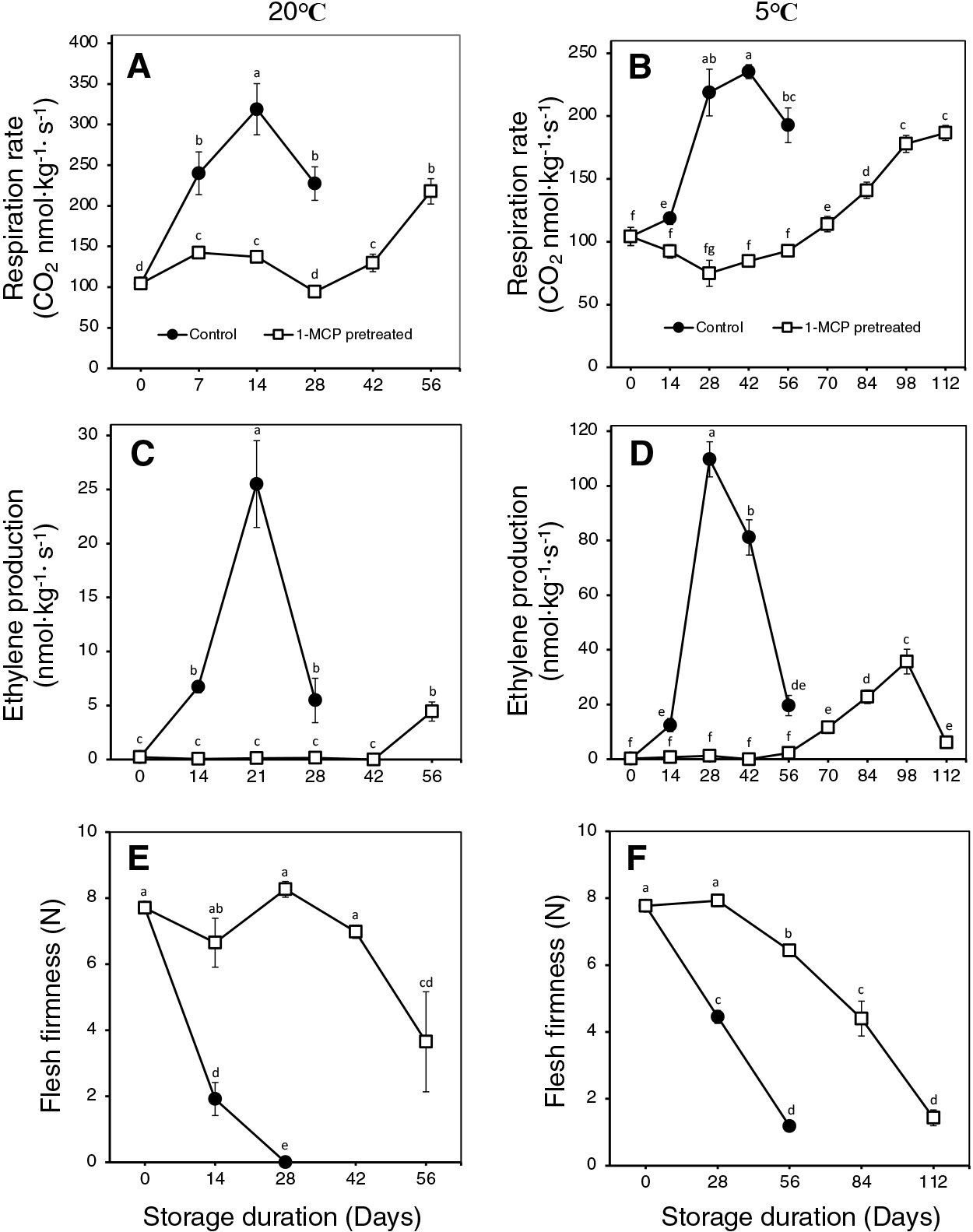
Respiration rates (A, B), ethylene production rates (C, D), and flesh firmness (E, F) of 1-MCP pretreated and non-treated (control) ‘La France’ pear fruit during storage at 20°C and 5°C. Values for respiration and ethylene production rates are means (± SE) of 10 individual biological replications (10 fruit). Flesh firmness values are means of three fruit. Different letters indicate significant differences in ANOVA (Tukey’s test, P < 0.05).
Non-treated control fruit also showed increased respiration and ethylene production rates during storage at 5°C; CO2 production rates reached the highest levels (220–235 nmol·kg−1·s−1) at 28 and 42 d (Fig. 1B), while peak ethylene production levels (110 nmol·kg−1·s−1) were recorded at 28 d (Fig. 1D). It is worth mentioning that non-treated fruit at 5°C produced appreciably higher ethylene levels than non-treated fruit at 20°C. In accordance with the increased respiration and ethylene production rates, flesh firmness of non-treated fruit decreased sharply to 4.5 N at 28 d and 1 N at 56 d (Fig. 1F). In 1-MCP-pretreated fruit, respiration rates were maintained at at-harvest levels for 56 d (Fig. 1B), after which a gradual and steady increase was observed. Ethylene production rates of 1-MCP-pretreated fruit were also maintained at minimal at-harvest levels for 42 d (Fig. 1D), but a gradual and steady increase started at 56 d reaching peak levels (35 nmol·kg−1·s−1) at 98 d. Finally, flesh firmness of 1-MCP-pretreated fruit remained unchanged for 28 d (Fig. 1F), but then decreased gradually afterwards reaching 1.4 N at 112 d. Both 1-MCP-pretreated and control fruit showed insignificant changes in SSC and TA during storage at 5°C (Supplementary Fig. 1B, D).
Taken together, these findings indicated that cold storage (at 5°C) alone could extend storage life in ‘La France’ pears to 56 d, but a single 1-MCP pretreatment extended the storage life further to 112 d. Additionally, 1-MCP-pretreated fruit at 5°C gradually softened and reached eating quality, which could eliminate previous ripening blockage concerns for ‘La France’ pears.
Overview of RNA-Seq analysisTo further understand the mechanisms for fruit ripening inhibition and subsequent recovery in 1-MCP pretreated ‘La France’ fruit, we carried out pairwise comparisons of RNA-Seq data to find significantly up- and down-regulated genes. By using the criteria described earlier in section 2.4, a total of 6,427 DEGs were detected and further delineated into eight clusters of co-expressed genes (Fig. 2; Supplementary Table 1). A majority of the DEGs belonged to cluster II (58%), followed by cluster VI (21.6%), cluster I (8.2%), cluster VIII (5.2%), cluster V (2%), cluster IV (1.9%), cluster III (1.8%), and cluster VII (1.3%).

Hierarchical clustering and heat map visualization of co-expressed genes in 1-MCP pretreated and non-treated ‘La France’ pear fruit during storage at 20°C and 5°C. The left panel represents the eight different modules (clusters of highly inter-connected genes) identified by WGCNA.
Cluster II mostly comprised downregulated genes. During storage at 20°C, these genes were downregulated in control fruit at 14 d, but their downregulation in 1-MCP-treated fruit started at 56 d. At 5°C, cluster II genes were downregulated in control fruit both at 28 and 56 d, but their downregulation in 1-MCP-treated fruit was slight at 56 d, and becoming intense at 84 d. Genes in cluster I were slightly upregulated during storage at 20°C; in control fruit at 14 d, and in 1-MCP treated fruit at 56 d. Notably, the upregulation of cluster I genes was more intense during storage at 5°C, particularly at 56 d, in control fruit and 84 d in 1-MCP-treated fruit.
Genes in clusters IV, V, and VII were upregulated during storage at 20°C; at 14 d in control fruit, and at 56 d in 1-MCP-treated fruit. During storage at 5°C, cluster IV genes were upregulated at 56 d in control fruit while their expressions increased gradually and steadily at 28, 56, and 84 d in 1-MCP-treated fruit. On the other hand, at 5°C, cluster V genes were upregulated at 28 and 56 d in control fruit, but only started to increase their expressions at 84 d in 1-MCP-treated fruit. Also, at 5°C, cluster VII genes were upregulated in control fruit at both 28 d and 56 d, while their expressions in 1-MCP-treated fruit started to increase at 56 and 84 d.
Genes that constituted clusters III and VIII were downregulated during storage at 20°C. In control fruit, genes of both clusters were downregulated at 14 d. However, in 1-MCP-treated fruit, cluster VIII genes were downregulated at 42 and 56 d, while cluster III genes were downregulated at 56 d. During storage at 5°C, genes of both clusters were downregulated at 28 and 56 d in control fruit, and at 84 d in 1-MCP-treated fruit.
Finally, the expression of cluster VI genes remained low and unchanged during storage at 20°C both in control and 1-MCP-treated fruit. However, these genes were upregulated during storage at 5°C; at 28 and 56 d in control fruit, and mostly at 56 and 84 d in 1-MCP-treated fruit. It should also be noted that some cluster VI genes were also upregulated earlier (at 28 d) in 1-MCP treated fruit.
Expression of genes encoding ethylene biosynthetic enzymesAs ethylene is quite instrumental in the control of fruit ripening in climacteric fruits, including European pears, we examined the expression patterns of key biosynthetic genes from the RNA-Seq data (Fig. 3). Notably, the expression levels of ACS1 (pycom01g09960) increased slightly in control fruit after 14 d during storage at 20°C, and after 56 d during storage at 5°C (Fig. 3A). However, expression levels were unchanged in 1-MCP-treated fruit both at 20°C and 5°C. ACS1b (pycom15g26520) expression levels showed no changes during storage at 20°C (Fig. 3B), but there was a dramatic increase during storage at 5°C, especially in control fruit at 28 and 56 d. In 1-MCP-treated fruit at 5°C, ACS1b expression was initially maintained at minimal levels for 28 d, but then increased slightly at 56 d, and sharply at 84 d. Both ACO1 (pycom05g32140) and ACO2 (pycom10g27800) displayed increased expression levels during storage at 20°C (at 14 d in control fruit and at 56 d in 1-MCP-treated fruit), as well as during storage at 5°C (at 28 and 56 d in control fruit, and at 56 and 84 d in 1-MCP treated fruit) (Fig. 3C, D).
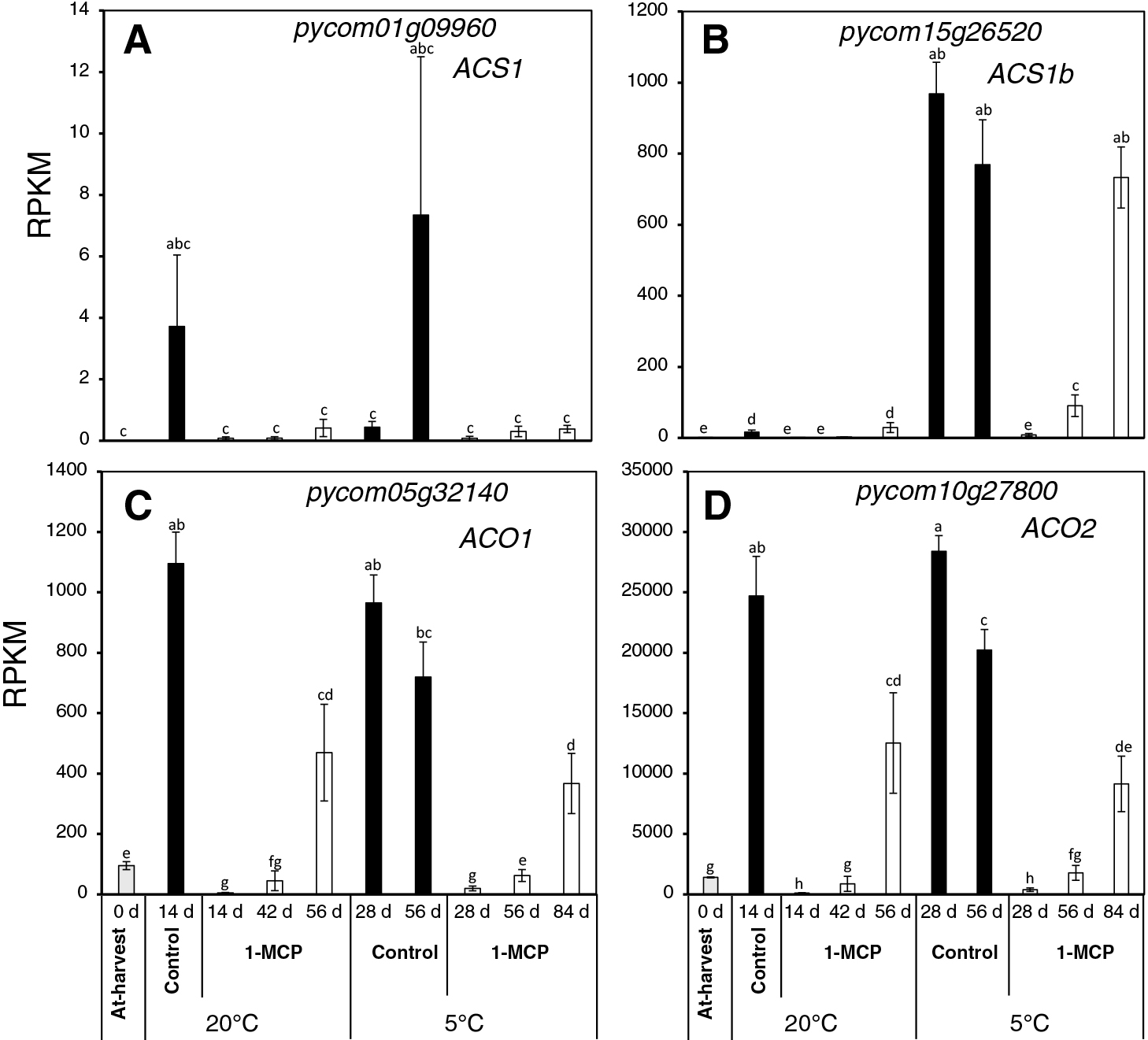
Expression levels of genes encoding ethylene biosynthetic pathway enzymes during storage of 1-MCP pretreated and non-treated (control) ‘La France’ pear fruit at 20°C and 5°C. RPKM—Reads per kilobase million. Values are means (± SE) of three individual biological replications (three fruit). Different letters indicate significant differences in ANOVA (Tukey’s test, P < 0.05).
The expression patterns of four genes that encode cell wall degrading enzymes which cause flesh softening, including PG3 (pycom03g19680), β-GAL (pycom15g18320), EG (pycom11g16540), and EXP1 (pycom14g16740), were also examined (Fig. 4). PG3 and β-GAL displayed quite similar expression patterns; during storage at 20°C, they were upregulated in control fruit at 14 d, and in 1-MCP-treated fruit at 56 d (Fig. 4A, B). During storage at 5°C, both PG3 and β-GAL were also upregulated in control fruit at 28 and 56 d, and in 1-MCP-treated fruit at 56 and 84 d. The expression levels of EG increased sharply in control fruit at 20°C, but in 1-MCP-treated fruit, they were slightly suppressed, and a gradual increase was observed at 14, 42, and 56 d (Fig. 4C). EG expression levels also increased during storage at 5°C in control fruit at 28 and 56 d, but the levels were considerably lower in 1-MCP-treated fruit. Lastly, EXP1 expression levels remained unchanged during storage at 20°C (both in control and 1-MCP-treated fruit), but significantly increased during storage at 5°C in control fruit, particularly at 28 and 56 d (Fig. 4D). In 1-MCP-treated fruit at 5°C, EXP1 expression levels only started to increase at 84 d.

Expression levels of genes encoding cell wall degrading enzymes, which are involved in flesh softening, during storage of 1-MCP pretreated and non-treated (control) ‘La France’ pear fruit at 20°C and 5°C. RPKM—Reads per kilobase million. Values are means (± SE) of three individual biological replications (three fruit). Different letters indicate significant differences in ANOVA (Tukey’s test, P < 0.05).
Genes encoding various TF families were among the DEGs identified by RNA-Seq analysis (Supplementary Table 1). We examined the expression patterns of agamous-like 18 (AGL18, pycom14g06350) and NAC29 (pycom11g10980), which belong to agamous MADS-box and NAC families that have been shown to regulate a wide range of plant growth and developmental processes, including fruit ripening (Itkin et al., 2009; Kou et al., 2021). As indicated in Figure 5A, the expression of AGL18 increased during storage at 20°C after 14 d in control fruit, and at 56 d in 1-MCP-treated fruit. AGL18 expression also increased during storage at 5°C at 28 and 56 d in control fruit, and at 84 d in 1-MCP-treated fruit. On the contrary, the expression levels of NAC29 were minimal and unchanged during storage at 20°C both in control and 1-MCP-treated fruit. However, there was a substantial increase in NAC29 expression during storage at 5°C in control fruit at 28 and 56 d, and at 84 d in 1-MCP fruit.
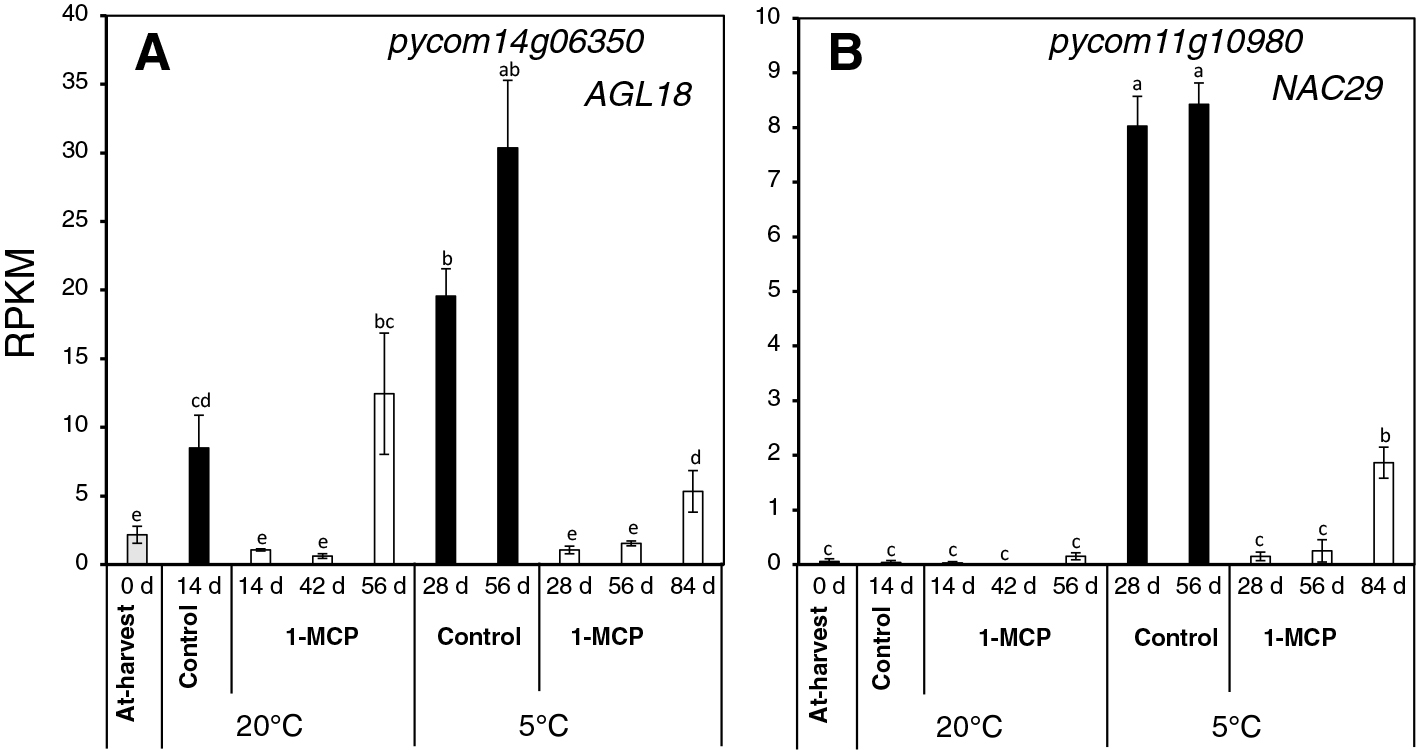
Expression levels of genes encoding selected transcription factors during storage of 1-MCP pretreated and non-treated (control) ‘La France’ pear fruit at 20°C and 5°C. RPKM—Reads per kilobase million. Values are means (± SE) of three individual biological replications (three fruit). Different letters indicate significant differences in ANOVA (Tukey’s test, P < 0.05).
Flesh softening that accompanies fruit ripening is important for palatability and consumer acceptability, but it also exacerbates mechanical damage and pathogen/disease attack during transportation and handling processes (Meli et al., 2010; Yang et al., 2017). In the present study, 1-MCP pretreatments prior to storage at both 20°C and 5°C markedly inhibited fruit ripening in ‘La France’ pears (Fig. 1), demonstrating its potential usefulness in the extension of postharvest life. The suppression of fruit respiration and ethylene production rates (Fig. 1A–D), and delay in flesh firmness decrease following 1-MCP treatment agrees with findings of previous research with other European pear cultivars (Argenta et al., 2003; Xie et al., 2014), and several other climacteric fruit (Watkins, 2006). During storage at 20°C, the flesh firmness of 1-MCP pretreated ‘La France’ fruit remained at high at-harvest levels for 42 d, and although it decreased afterwards, the fruit were considerably firmer at 56 d than non-treated fruit, which attained eating quality firmness at 14 d. During storage at 5°C, the flesh firmness of 1-MCP pretreated fruit gradually decreased to eating quality at 112 d, compared to non-treated fruit that attained eating quality at 56 d. These findings demonstrate that simple one-time 1-MCP pretreatments can extend postharvest life in ‘La France’ fruit for up to 56 d during shelf life storage, and 112 d in the case of cold storage.
Fruit ripening recovery after 1-MCP treatmentBefore commercial application of 1-MCP, it is important to consider the time it takes for treated fruit to start ripening normally. This is particularly important for fruit that require ethylene to attain optimal consumption quality attributes such as softening, emission of aroma volatiles, and development of attractive color. Fruit ripening recovery following 1-MCP treatment was found to occur after 2 d in mangoes (Li et al., 2020), 3 d in tomatoes (Opiyo and Ying, 2005), and 4 d in kiwifruit (Mworia et al., 2010). However, in European pears, studies in several cultivars have shown that 1-MCP treatment irreversibly inhibits fruit softening (Argenta et al., 2003; Chiriboga et al., 2010; 2011; Villalobos-Acuña and Mitcham, 2008), thus limiting the practical use of 1-MCP to extend storage life. In the present study, ‘La France’ pears treated with 1-MCP regained their softening capacity and softened to eating quality firmness beyond 42 d at 20°C (Fig. 1E). During cold storage, 1-MCP-treated ‘La France’ fruit also displayed ripening recovery, gradually softening to a desirable eating quality firmness after 112 d (Fig. 1F). Therefore, unlike in other European pear cultivars, real-life application of 1-MCP to extend postharvest life in ‘La France’ fruit seems feasible as the above findings mitigate the previous irreversible ripening blockage.
Transcriptomic changes involved in 1-MCP-induced fruit ripening inhibition and subsequent recovery‘La France’ pears are climacteric fruit, and their ripening is triggered and regulated largely by ethylene (Hiwasa et al., 2003). In the present study, RNA-Seq analysis revealed that many genes were either up- or down-regulated in non-treated fruit both at 20°C and 5°C (Fig. 2). These gene expression changes corresponded with the presence of ethylene (Fig. 1E, F), indicating that ethylene was involved in their regulation. Indeed, 1-MCP pretreatment completely abolished the above gene expression changes, and their differential expression occurred only when 1-MCP- treated fruit started to produce ethylene. 1-MCP has been shown to irreversibly bind to ethylene receptors, resulting in stable complexes that suppress ethylene signaling (Kamiyoshihara et al., 2012). Therefore, it is clear that 1-MCP treatment inhibited fruit ripening in ‘La France’ essentially by delaying the onset of autocatalytic ethylene production, which is characteristic of climacteric fruit ripening.
Low temperature has been shown to trigger fruit ripening in several European pear cultivars by activating autocatalytic ethylene production (Villalobos-Acuña and Mitcham, 2008). In fact, fruit of late-maturing cultivars such as ‘Passe Crassane’ require low temperature treatments before they can attain ripening capacity (El-Sharkawy et al., 2004; Mitalo et al., 2019a). In the present study, ‘La France’ fruit produced ethylene and ripened normally during storage at 20°C (Fig. 1C, E), indicating that they do not necessarily require low temperature to ripen. However, ethylene production rates were noticeably higher during storage at 5°C than at 20°C (Fig. 1D), which is suggestive of a “magnification effect” of low temperature on ethylene biosynthesis. This magnification effect of low temperature in European pears was found to be two-fold; first by augmenting already existing ethylene responses, and second, by emergence of new ethylene responses (Mitalo et al., 2019a), and it is further echoed by the RNA-Seq data in this study. Cluster I genes showed only a slight response to ethylene at 20°C (Fig. 2), but their upregulation was massive at 5°C. Furthermore, cluster VI genes failed to respond to ethylene during storage at 20°C, but their ethylene responsiveness appeared during storage at 5°C. Therefore, it appears that an unknown change in the physio-molecular state of fruit during cold storage could increase sensitivity to ethylene. Future research should further clarify this point.
It has also been broadly demonstrated that low temperature promotes fruit ripening independently of ethylene in several fruit types including kiwifruit and lemons (Asiche et al., 2018; Mitalo et al., 2019b, 2020). This was mostly based on findings that certain fruit ripening-associated factors and corresponding genes were accelerated during cold storage, while repeated 1-MCP treatments failed to suppress the changes. In the present study, 1-MCP treated fruit at 5°C started to soften long before ethylene production was detected (Fig. 1D, F), suggesting that ethylene-independent ripening could also exist in ‘La France’ pears. Ethylene-independent induction of fruit softening was also reported in ‘Royal Gala’ apples (Tacken et al., 2010), and several low temperature-regulated genes in ‘Bartlett’ and ‘Passe’ Crassane’ pears were found to be ethylene-independent (Mitalo et al., 2019a; Nham et al., 2017).
Ethylene biosynthesis is correlated by the activities of ACS and ACO enzymes, both of which are encoded by multigene families (Cherian et al., 2014; Wang et al., 2002). In European pears, it has been demonstrated that several ACS and ACO genes are expressed during fruit ripening, and their transcript levels increased in response to ethylene and/or low temperature (El-Sharkawy et al., 2004; Fonseca et al., 2005; Xie et al., 2014). Based on RNA-Seq analysis, we confirmed that transcription of ACS1, ACO1, and ACO2 was highly stimulated both at 20°C and 5°C (Fig. 3A, C, D), and expression was maintained at minimal levels in 1-MCP-treated fruit pending the start of ethylene production. The expression of ACS1b was low throughout storage at 20°C (Fig. 3B), but increased during storage at 5°C in non-treated fruit after 28 and 56 d, and in 1-MCP-treated fruit after 56 and 84 d. The high and exclusive stimulation of ACS1b expression at 5°C could account for the higher ethylene production rates at 5°C than at 20°C (Fig. 1C, D), given that ACS genes regulate the rate-limiting step in ethylene biosynthesis (Wang et al., 2002; Yip et al., 1992).
Reduction in fruit firmness during ripening is largely due to increased expression of cell wall degrading enzymes that act on proteins and carbohydrates in primary cell walls (Wang et al., 2019; Xiao et al., 2019). Several genes encoding these cell wall degrading enzymes including PGs, β-GALs, EGs, and EXPs, were shown to be regulated by ethylene (Bennet and Labavitch, 2008; Mwaniki et al., 2021), and their stimulation by low temperature has also been demonstrated in European pears (Fonseca et al., 2005; Mitalo et al., 2019a). In the present study, the data show that PG3, β-GAL, and EG transcription was stimulated in non-treated fruit both at 20°C and 5°C (Fig. 4A–C), which correlated well with reductions in flesh firmness (Fig. 1E, F). However, the transcription of EXP1 was only stimulated during storage at 5°C (Fig. 4D), indicating that its expression relies heavily on low temperature. Nevertheless, all four genes examined were inhibited by 1-MCP treatment, and their expressions increased only after treated fruit started to produce ethylene, confirming that they are regulated by ethylene.
Prior studies have shown the importance of AGL MADS-box and NAC TFs in fruit development and ripening. In tomato, TAGL1 expression was found to increase during fruit ripening and a TAGL1 loss-of-function mutant showed lower expression of fruit ripening-associated genes (Itkin et al., 2009). Several NACs were also shown to regulate fruit ripening by direct interaction with fruit ripening-associated genes in different types of fruit (Kou et al., 2021). In the present study, AGL18 transcript levels increased in non-treated fruit both at 20°C and 5°C as well as in 1-MCP-treated fruit after ethylene production was detected (Fig. 5A), suggesting its involvement in regulating fruit ripening. On the other hand, NAC29 was only upregulated during storage at 5°C and 1-MCP inhibited its upregulation until the treated fruit started to produce ethylene (Fig. 5B). This finding also suggests that NAC29 regulation by ethylene is mediated by low temperature.
ConclusionsFrom the data presented in the current study, it is clear that a one-time postharvest application of 1-MCP significantly delayed the onset of fruit ripening both at 20°C and 5°C in ‘La France’ pears by suppressing ethylene production and respiration rates. Unlike previous reports in other European pear cultivars, 1-MCP pretreated ‘La France’ pears regained their fruit softening capacity and attained eating quality firmness after 56 d at 20°C and 112 d at 5°C. Fruit ripening inhibition by 1-MCP and subsequent recovery were clearly supported by the expression patterns of various associated genes as revealed by RNA-Seq analysis. Therefore, 1-MCP has potential for commercial use as a simple technique to extend postharvest life in ‘La France’ pear fruit after preliminary tests to find the optimum concentration and storage temperature have been carried out.
This study was supported by a Grant-in-Aid for Scientific Research (grant no. 20H02977) from the Japan Society for the Promotion of Science, Japan.