2023 年 92 巻 2 号 p. 115-124
2023 年 92 巻 2 号 p. 115-124
Young coconut shell cracking is a significant problem reported during trimming and polishing. By studying the shell structure, fruit development, pressure inside the shell (internal pressure) of young fruits, dissolved carbon dioxide in the coconut water and cracking incidence, as well as postharvest treatments to manipulate the pressure and carbon dioxide concentration, it was revealed that cracking depended on the physical property of the shell and the internal pressure. Cracking was principally found on the large carpel where the shell was the thinnest. The growing area presenting a high cracking incidence was found to have fruits with a thinner shell than fruits from a nearby area. The younger fruits had a weaker, thinner shell and high internal pressure. Once the fruits were 28 weeks old, the internal pressure dropped to slightly above atmospheric pressure. The level of internal pressure did not depend on rainfall or growing area. However, postharvest heating, dipping in water and exposure to carbon dioxide increased the internal pressure. To reduce shell cracking incidence, it is recommended to delay harvesting to allow shell strengthening, delay the trimming and polishing processes to allow water loss from the fruit and reduce the internal pressure. Dipping fruit in an anti-browning solution should also be brief to avoid water absorption.
Young coconut (Cocos nucifera) is popular for its refreshing liquid content and tender kernel. The export value of young coconuts from Thailand attained 283 million USD in 2021 (Office of Agricultural Economics, 2022). It is produced from the dwarf-type coconut tree. In one year, each tree generally produces approximately 18 inflorescences, one every three weeks. In one inflorescence, there are about 50 female but thousands of male flowers. After pollination with the assistance of insects, the fruit set is less than 50% of the female flowers. Only 10 to 20 fruits per bunch survive until the fully mature stage, 11 months after full bloom. The young fruits are typically harvested seven months after full bloom (Siriphanich et al., 2011). The main consumer markets are in China and the USA, predominantly as trimmed and polished fruit. Trimming away a portion of the husk to make a diamond shape fruit reduces the fruit weight by one-third. Polishing the fruit by removing most of the husk except that covering the soft eye, one of the three circular scars on the stem end of the coconut shell where the embryo is located, further reduces the fruit weight by two-thirds. However, polished coconut has a short storage life of about 2–3 weeks, limited by mold growth on the husk covering the soft eye, even though sodium metabisulfite is used to control browning and mould growth. Another problem during or after trimming the husk and polishing the shell is shell cracking (Siriphanich et al., 2011), as shown in Figure 2C.
In other fruits, many previous studies have focused on factors involving cracking, and most were in relation to the soft tissue of the exocarp and mesocarp (Byers et al., 1990; Cline and Tehrani, 1973; Huang et al., 2005, 2008; Khanal et al., 2011; Peet, 1992; Simon, 2006; Tongumpai, 1993). Only a few studies were performed on internal cracking of the shell or hardened endocarp and clarified the causes or mechanisms. For example, split-pit in peaches may develop when the pit is still soft and the merging at the cleavage site of the endocarp is incomplete (O’Malley and Proctor, 2002). Moreover, hard thinning of fruit induced mass flow of photosynthates from source to sink (fruit), causing an increase in flesh growth. Increasing the flesh growth raises the radial tensile force and eventually causes cracking of the fruit stone (Nakano and Nakamura, 2002). Recently, Drogoudi and Pantelidis (2022) studied 59 cultivars of peach and nectarine over four years and concluded that early ripening was the most important parameter related to a higher percentage of fruit with split-pit, followed by larger fruit and higher rainfall 30 days after blooming. These studies suggested that endocarp strength and internal pressure were two important factors involved in endocarp cracking in peaches.
Shell cracking in de-husked mature coconuts imported into Mexico was reported at 5–10% (Bruton, 1982). In the study, cracked positions (referred to as stress cracks) were predominantly found in a transverse direction on the distal half of the endocarp. A cracked grove was usually found only at the endocarp and not at the solid endosperm. Furthermore, weight loss was found to be strongly related to mature coconut cracking. Coating mature coconut with paraffin or carnauba wax reduced shell cracking by 97% and 60% over the control, respectively.
Scholander (1955) reported that young coconut fruit had high internal pressure, but the pressure decreased as the fruit became mature. Shell cracking of young, polished coconuts is often reported in the rainy season. It is considered that more water is absorbed into the fruit during the rainy season, causing a higher internal pressure compared to the dry season.
In addition, during the opening of the young coconut shell for consumption, coconut water and gas often rushes forcefully out of the fruit, comparable to opening a shaken soda bottle. Since coconuts accumulate lipid in their solid endosperm, it is possible that carbon dioxide, which is the byproduct of fatty acid biosynthesis (Ohlrogge et al., 2015), dissolved in coconut water could be another source of internal pressure that causes shell cracking. Moreover, since young coconuts are harvested when the fruit has not fully developed, the shell is not hardened, and its natural protection is lost as the husk is removed. Hence, another possible factor influencing the cracking of young coconut is shell strength, similar to the split-pit reported in peaches when the pit is still soft (O’Malley and Proctor, 2002).
We hypothesized that young, polished coconut fruits crack when the shell is weak and thin, internal pressure is high, and carbon dioxide concentration in the coconut water is high. Our objective was to verify these hypotheses by investigating fruits harvested at different maturity stages, seasons, growing areas, and under different post-harvest conditions and to recommend solutions to avoid cracking.
Young fruit of dwarf type coconut (Cocos nucifera L.) cv. ‘Nam Hom’ were obtained from orchards in Damnoen Saduak district, Ratchaburi province, Thailand, and the adjacent Ban Phaeo district, Samut Sakhon province. The trees were 10–17 years of age, grown on 5-m broad ridges, and with 2-m wide water furrows on both sides. The water level was maintained at approximately 30 cm below the top of the ridge throughout the year. The inflorescences were tagged at full bloom. Fruits were harvested in the morning and brought to the laboratory within 2 h.
Experiments (a) Shell properties in relation to cracking (i) Shell thickness at different positions of the fruitThirty fruits at 28 ± 1 weeks after full bloom (WAF) were randomly selected, one fruit from one bunch, harvested from an orchard in Damnoen Saduak, trimmed, and polished. The fruits were then measured for shell thickness around the fruit equator and in the longitudinal direction from the middle of the most prominent carpel stem end to the stylar end using a digital Vernier caliper (103 INCH; Mitutoyo, Kanagawa, Japan).
(ii) Cracking position and properties of the shell from cracked and non-cracked fruitsPolished coconuts at 28 ± 2 WAF were sampled from a packinghouse in Ban Praew in January, March, and May 2012 (total of 1,500 fruits per month). The number of cracked fruits were counted, and the cracking position of each fruit was also recorded, whether it was on the large carpel or small carpel and from the upper, middle, or lower third of the fruit. Cracking percentages of total fruit were calculated.
Ten cracked and non-cracked fruits were randomly collected from each harvest. A square chip of the shell (3 × 3 cm) together with the white kernel (solid endosperm) was cut out from the middle of the biggest carpel midway between the equator and the stylar end of each fruit and used for measuring the shell and kernel thickness using a Vernier caliper. The shell was collected, weighed, and then dried in a hot air oven at 60°C for three days to determine shell water content.
(b) Temporal variation of internal pressure and cracking rate of coconuts (i) Monthly variation in pressure and cracking rate During a two-year period from one growing locationTen uniform trees in Damnoen Saduak, Ratchaburi province, were selected. Seven to ten bunches of coconuts at 28 ± 1 WAF were harvested monthly from these trees over two years, from September 2013 to August 2015. At each harvest, all the fruits were trimmed and polished to remove the husk within 3 h of harvesting. Three polished fruits were immediately and randomly taken from each bunch to measure the pressure inside the shell (see section 3 for method). The shell cracking rate was determined from the rest of the polished nut 24 h later. Weather data during the fruit development were obtained from the Ratchaburi Meteorological Station.
During a one-year period from two growing locationsSimilar to above, coconut fruits were harvested from 2019 to 2020 from two commercial orchards in Damnoen Saduak, Ratchaburi province, and Ban Phaeo, Samut Sakhon province. The fruits were determined for internal pressure, shell cracking rate, and CO2 concentration in the coconut water (see Section 3 for method). Weather data in Damnoen Saduak and Ban Phaeo were obtained from the Ratchaburi Meteorological Station and Ban Phaeo Irrigation Project Station, respectively. The relation between the internal pressure and shell cracking percentage was analyzed using linear regression and Pearson’s correlation methods.
(c) Internal pressure and CO2 concentration during fruit growthFive uniform coconut trees were selected from an orchard in Damnoen Saduak. As new inflorescences developed approximately every three weeks, full-bloom inflorescences were regularly tagged on all trees. Coconut bunches, at 8 to 40 WAF, were harvested concurrently in November 2019. Three to five bunches were harvested from five trees since not all the trees produced new inflorescences in sync. Three fruits were randomly collected from the three bunches for internal pressure and dissolved CO2 concentration measurements. The two extra bunches were discarded.
The internal pressure of each coconut fruit was determined within 2–3 h of harvesting. After the internal pressure reading, the coconut water was collected into 15 mL vials, and sealed with a Teflon septum and aluminum cap for dissolved CO2 concentration determination.
(d) Effect of post-harvest factors on internal pressure and shell cracking (i) Effect of dipping coconut underwater on internal pressure and cracking rateThree bunches of coconut at 28 ± 1 WAF were harvested from Damnoen Saduak. The fruit were removed from the bunches, randomly divided into three groups of ten, and trimmed and polished. The fruits of the first two groups were measured for internal pressure at 0 and 24 h after polishing. The third group was kept under ambient conditions for 24 h, dipped under water for 24 h, and then immediately measured for internal pressure. Fruits from another six bunches were also randomly divided into two groups of thirty. One group was kept under ambient condition, whereas the other was kept in water. All the fruits were determined for shell cracking incidence 24 h later.
(ii) Effect of heatingCoconuts at 28 ± 1 WAF were harvested from ten trees in Damnoen Saduak, one bunch from each tree. The fruits were detached from the bunches, mixed, then trimmed and polished to remove the husk except around the stem end, wrapped with polyvinyl chloride (PVC) film, and kept at room temperature or 40°C. After 0, 1, 2, and 4 h, ten fruits from both temperature regimes and durations were used for measuring internal pressure, dissolved CO2 concentration, and coconut water temperature.
(iii) Effect of atmospheric conditionCoconut fruits at 28 ± 1 WAF were harvested from four trees in Damnoen Saduak. Twenty-four fruits of uniform size were trimmed and polished. Six fruit were placed in each of three closed 14 L plastic chambers, with air, nitrogen, or carbon dioxide flowing through for 24 h. All the gases were humidified by bubbling through water before entering the chamber. After 24 h of different gas exposure, internal pressure and dissolved CO2 were measured in the fruits; the pressures were compared with the pre-treatment pressure. At the end of the experiment, carbon dioxide concentration in the control, nitrogen, and carbon dioxide chambers was found to be 3.2, 1.2, and 100%, respectively.
(e) Shell cracking prevention measures (i) Effect of delayed polishing timeFifteen bunches of coconut fruits at 28 ± 1 WAF were harvested from Damnoen Saduak in October 2019. The fruits were removed from the bunches, combined, and randomly divided into five groups, each group comprising thirty fruit. The fruits were subsequently trimmed and polished at 0, 3, 6, 12, and 24 h after harvesting. Ten fruits from each group were measured for internal pressure and dissolved CO2 concentration immediately after polishing. The other twenty fruits were kept under ambient conditions (25 ± 2°C, 73 ± 5% RH) for 24 h; then, the shell cracking percentage was recorded.
Pressure and carbon dioxide determination (a) Pressure measurementThe internal pressure of young coconuts was determined within 2–3 h of harvesting. The husk around the stylar end of the fruit was removed with a sharp knife. A hypodermic needle (size 24G), connected by a Tygon tube to pressure a measuring apparatus, penetrated the fruit through the soft eye of the large carpel. The piercing area was covered beforehand with an approximately a 1-mm layer of silicone grease to prevent the leakage of coconut water. The pressure was recorded in kPa. For 2013–2015, measurements were recorded using a custom-made manometer, as described in Figure 1. For 2019, measurements were recorded using a pressure meter (Testo-511; Testo AG, Lenzkirch, Germany). The pressure readings of the two measurement methods were comparable.
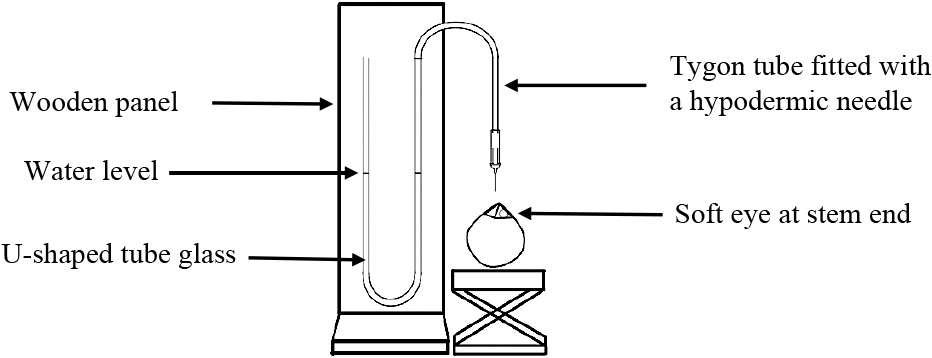
Diagram showing the tool used to measure internal pressure of young coconuts. The tool was built from a u-shape glass tube fixed on a wooden panel. The tube was filled with water and connected to a Tygon tube fitted with a needle (size 24G) at its end. The change in the water level inside the u-shaped glass tube was measured and used to calculated the internal pressure using the equation: Pcoconut = pgh + P0, where Pcoconut = Internal pressure of coconut, p = specific gravity of water (1,000 kg·m−3), g = acceleration of gravity (9.8 m·s−2), h = changing of water level (m), and P0 = atmospheric pressure (101.3 kPa).
Aliquots of coconut water were collected via syringe directly from the soft eye and transferred to 15-mL vials (1 mL aliquots for each of two vials from each fruit). The vial was sealed with a Teflon septum and an aluminum cap. The vials were heated in a 60°C water bath for 30 minutes. Gas samples were then withdrawn from both vials with a needle and syringe to determine the CO2 concentration by using a GC-8A Shimadzu gas chromatograph equipped with a Porapak Q column and a thermal conductivity detector. Dissolved CO2 concentration in the coconut water was calculated using the following equation: Dissolved CO2 (g·L−1) = [(% CO2 × (A−B) mL)/100] × [44 g/22,400 mL] × 1,000, where % CO2 corresponds to the CO2 concentration in the vial, A corresponds to vial gas volume (15 mL), B corresponds to coconut water volume in the vial (1 mL), 44 g corresponds to the weight of 1 gmol of CO2, and 22,400 mL corresponds to the volume of 1 gmol of gas.
Statistical analysisData on parameters measured during the study were subjected to analysis of variance (ANOVA), and means were separated using Tukey’s test (P ≤ 0.05). Pearson’s correlation coefficients were used to analyze the correlations among rainfall, average temperature, internal pressure, carbon dioxide concentration, and shell cracking. t-test was used for comparisons between two treatments (P ≤ 0.05). All data were subjected to statistical analysis using SPSS version 21.
The shell thickness measurement of polished coconuts along the fruit equator revealed that the thickest part of the shell was 3.16 mm at the ridge between the two small carpels, followed by the two ridges between the large and the two small carpels. The thinnest part, 2.02–2.16 mm, was on the biggest carpel, whereas it was between 2.53 and 2.68 mm on the two small carpels (Fig. 2A). The shell thickness along the length of the large carpel was highest at the stem end. The shell became gradually thinner toward the lower part of the nut. The thinnest part, 1.75 mm, was located midway between the equator and the stylar end. Below this, the shell became thicker again (Fig. 2B).
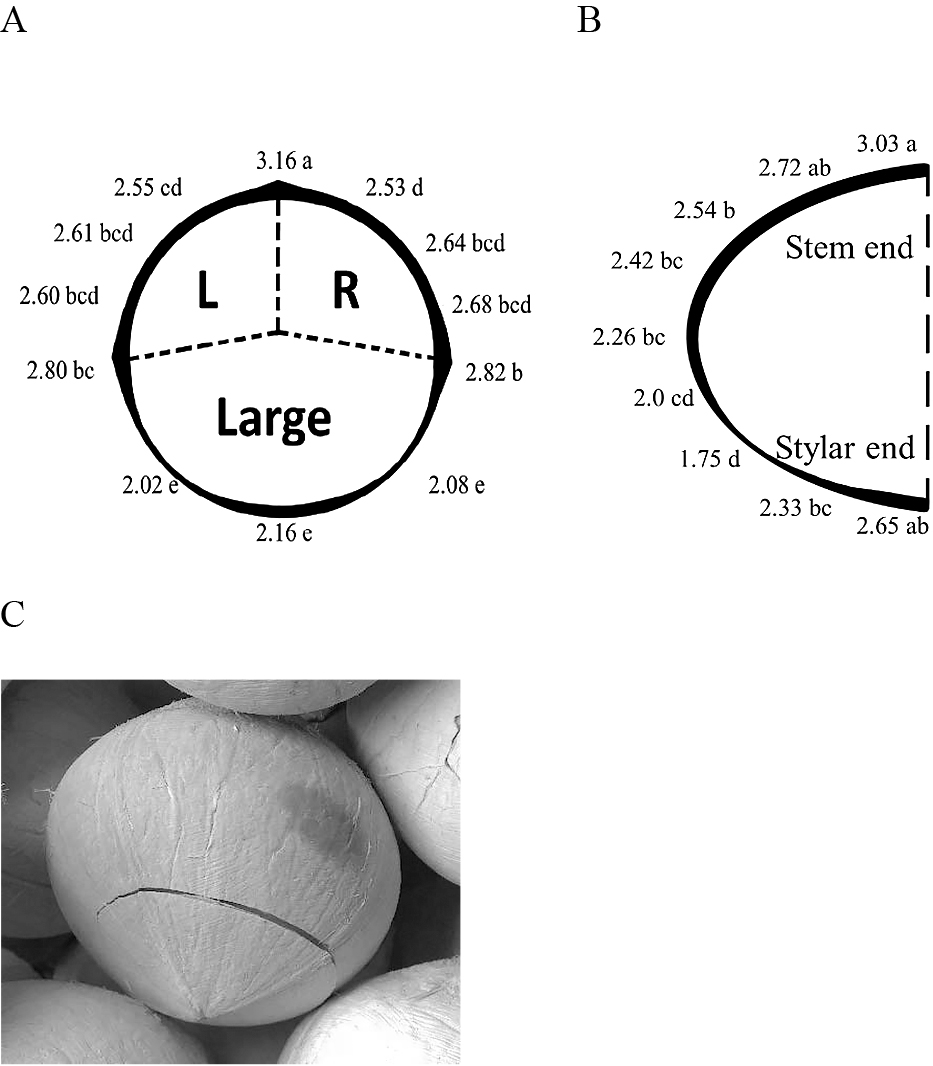
Shell thickness (mm) of young coconuts at 28 ± 1 WAF along the equator (A) and along the length of the biggest carpel (B). Cracking in young, polished coconuts (C). *Means followed with the same letter are not different based on Tukey’s test at P < 0.05 (n = 30).
Most cracking occurred as a transverse line parallel to the fruit circumference, in the large carpel, midway between the equator and the stylar end of the fruit (Fig. 2C; Table 1), where the shell in this position appeared to be thinnest (Fig. 2A, B).
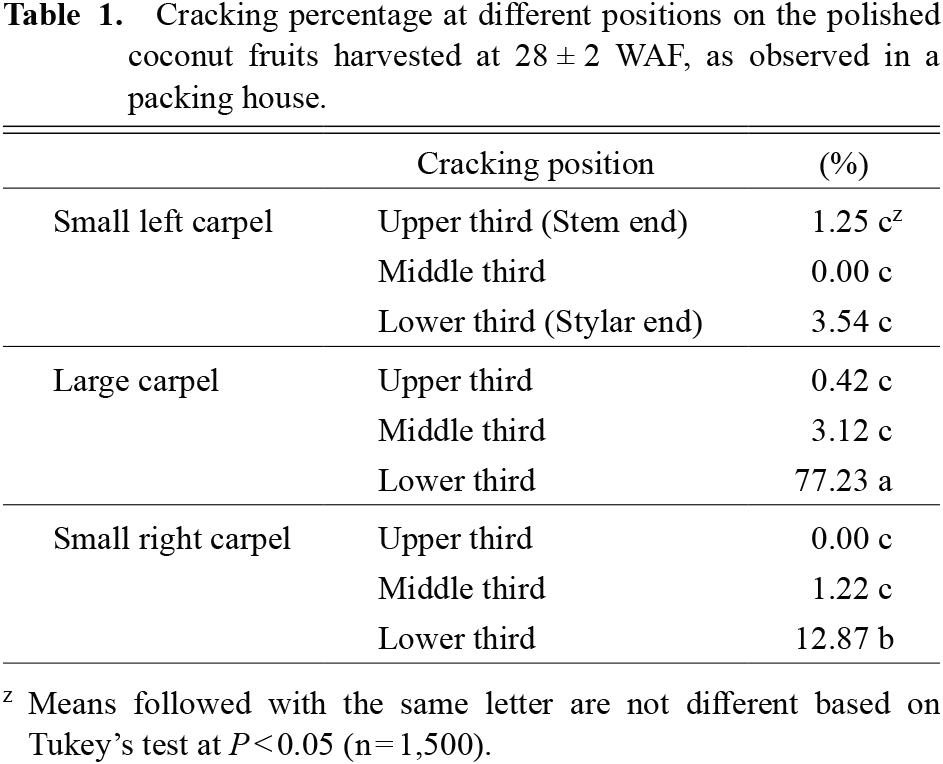
Cracking percentage at different positions on the polished coconut fruits harvested at 28 ± 2 WAF, as observed in a packing house.
When comparing the cracked and non-cracked fruits, it was found that the cracked fruits had a significantly thinner shell than the non-cracked fruits and had substantially higher water content. The cracked fruits also had a considerably thinner kernel (solid endosperm) than the non-cracked fruits (Table 2).

Shell thickness, shell water content, and kernel thickness of non-cracked and cracked polished young coconuts harvested at 28 ± 1 WAF.
During the two years in the growing area of Damnoen Saduak, high rainfall (average 144 mm·m−1) was recorded between May and October. In contrast, low rainfall (average 35 mm·m−1) was recorded between November and April each year (Fig. 3A). Average temperatures were high (31°C) between March and May, but were lower (24°C) in November and January (Fig. 3B). The internal pressure was slightly above the atmospheric pressure (101.5–102.7 kPa) throughout the two-year period (Fig. 3C), along with a low to moderate cracking incidence (0–6%) (Fig. 3D).

Rainfall (A) and average temperature in Ratchaburi (B) from September 2013 to August 2015; average internal pressure, n = 7–10 (C) and total cracking percentage 24 h after polishing, n = 50–70 (D) for each harvest. Bars indicate standard deviation.
No correlation was found between the accumulated rainfall 1, 3, 5, and 7 days before harvest date and the internal pressure. Conversely, rainfall was negatively related to shell cracking, particularly 5 and 7 days before harvesting (Table 3). No correlations were found among the average temperature, internal pressure, and shell cracking rate. Internal pressure and cracking shell percentage during the two-year study were also unrelated.
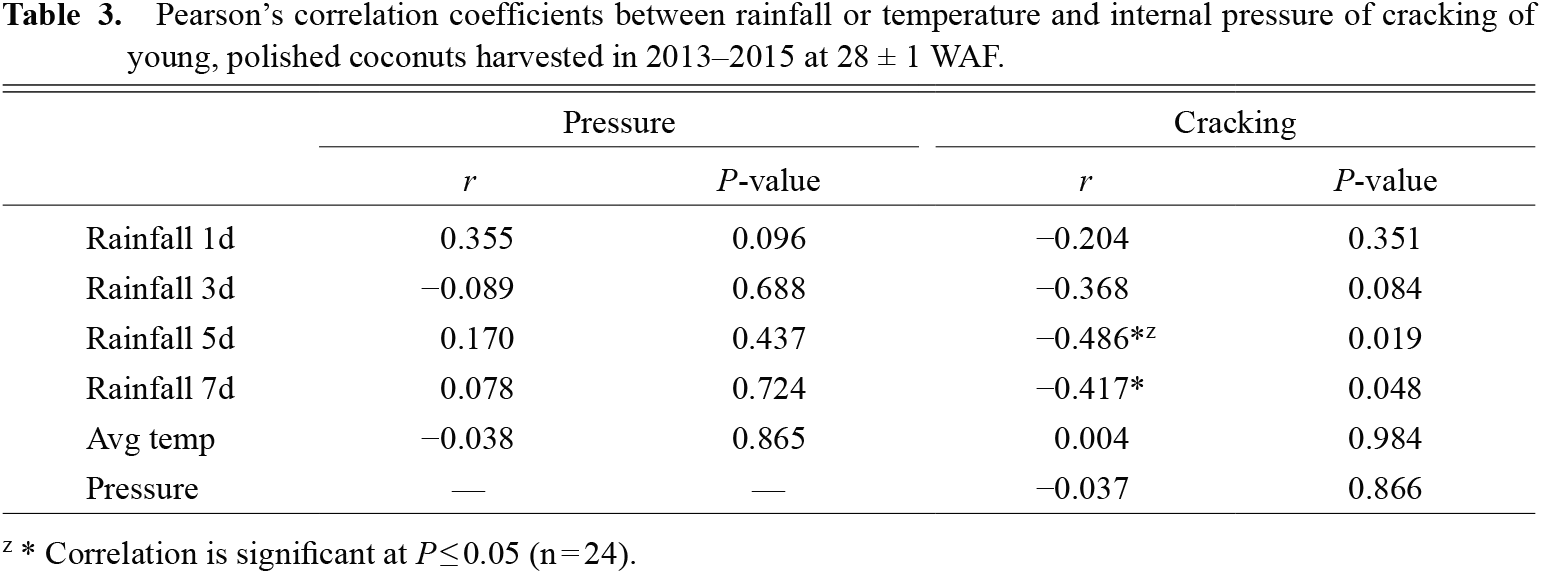
Pearson’s correlation coefficients between rainfall or temperature and internal pressure of cracking of young, polished coconuts harvested in 2013–2015 at 28 ± 1 WAF.
In the 2019–2020 season, rainfall level, distribution, and average daily temperature (Fig. 4A, B) were similar to that in the previous study. Fruits from both growing locations had high internal pressure (105–111 kPa) from April to June. The pressures in fruits from Damnoen Saduak were about 5 kPa lower than those in Ban Phaeo. In other months, the internal pressure was near atmospheric pressure, and no difference was found between the two locations (Fig. 4C). For CO2, relatively low concentrations (~0.6 g·L−1) were found in Damnoen Saduak in April and May 2019. The concentrations were higher (~0.7 g·L−1) from July to December but dropped to a low level from January to March 2020. For Ban Phaeo, the CO2 concentrations roughly followed the trend in Damnoen Saduak, except during August and September, where the CO2 concentrations were lower (Fig. 4C). A high rate of shell cracking was observed in fruits from Damnoen Saduak during May, August, and January, whereas in Ban Phaeo, a high rate of cracking was only found in April (Fig. 4D).
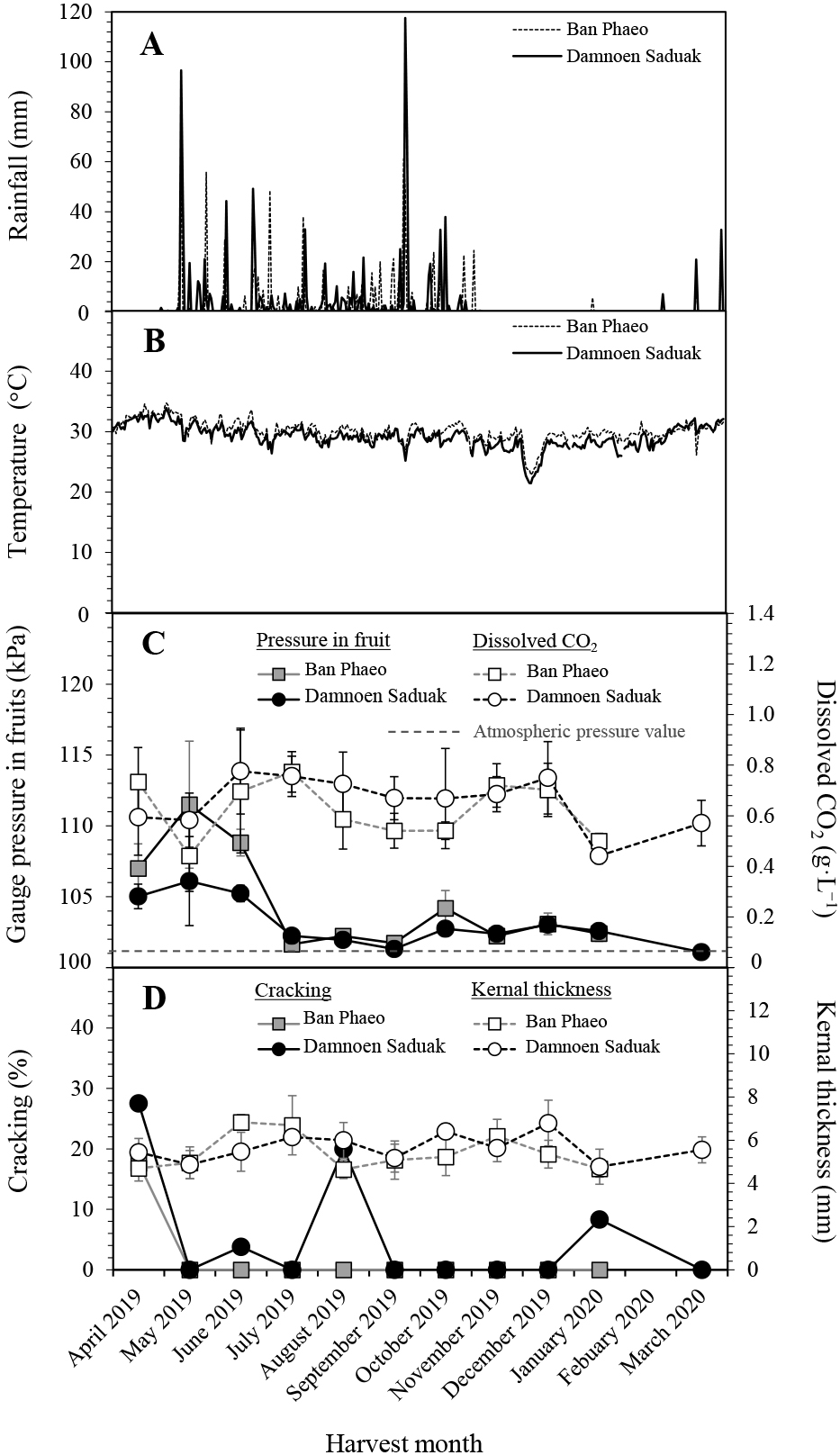
Rainfall (A) and average daily temperature (B) in Ban Phaeo and Damnoen Saduak growing locations, average internal pressure and dissolved carbon dioxide concentration in coconut water of intact coconut fruits, n = 7–10 (C), and total cracking percentage of fruits after the polishing process and kernel thickness, n = 50–70 (D), harvested at 28 ± 1 WAF from both locations. Bar on each data point indicates standard deviation.
Correlation analysis among the various parameters revealed predominantly no significant correlation (Table 4); only significance was identified between the average temperature, pressure, and cracking in the Ban Phaeo growing area.
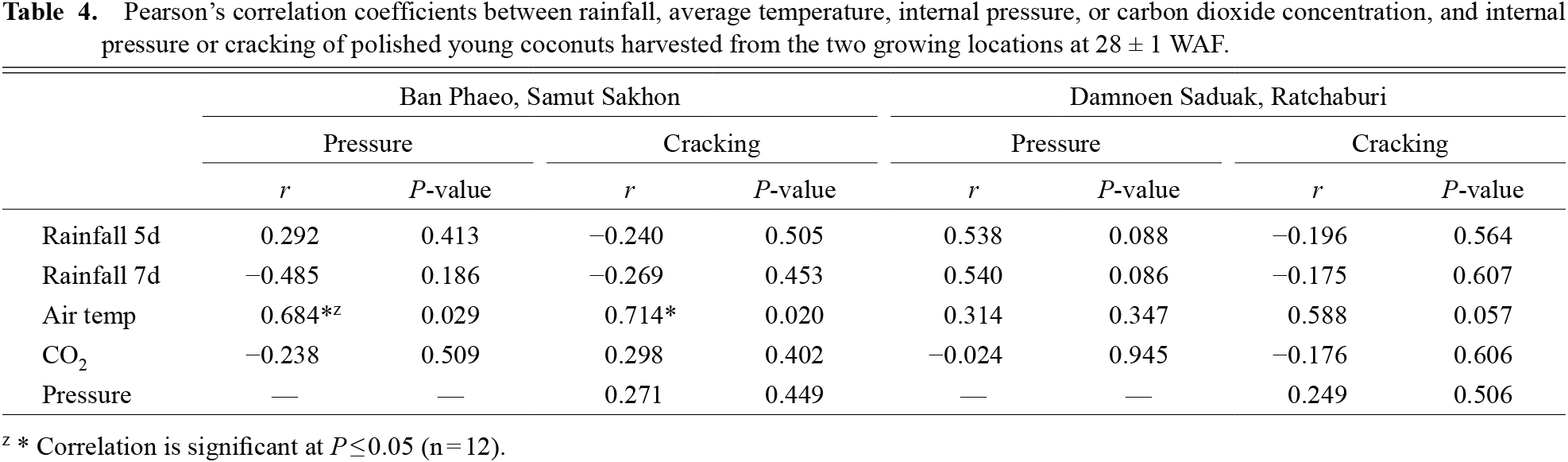
Pearson’s correlation coefficients between rainfall, average temperature, internal pressure, or carbon dioxide concentration, and internal pressure or cracking of polished young coconuts harvested from the two growing locations at 28 ± 1 WAF.
Regarding the physical properties of coconuts from the two locations, the fruits from Damnoen Saduak were significantly larger than those from Ban Phaeo, containing more water (298.9 versus 253.7 mL/fruit, P < 0.001), but had a thinner shell (2.17 verses 2.49 mm, P < 0.01).
Internal pressure and CO2 concentration during fruit growthThe water or liquid endosperm volume increased at 24 WAF during coconut fruit growth. In comparison, the kernel developed after 16 weeks and reached maximum thickness at 36 WAF (Fig. 5A). The internal pressure was very high during the first 24 weeks, after which the pressure dropped to slightly higher than the atmospheric pressure at 28 WAF and then remained stable (Fig. 5B). Conversely, the CO2 concentration in the coconut water was low at about 0.1 g·L−1 during the first 24 weeks, then abruptly increased to a maximum at 32 WAF, and declined slightly toward fruit maturity.
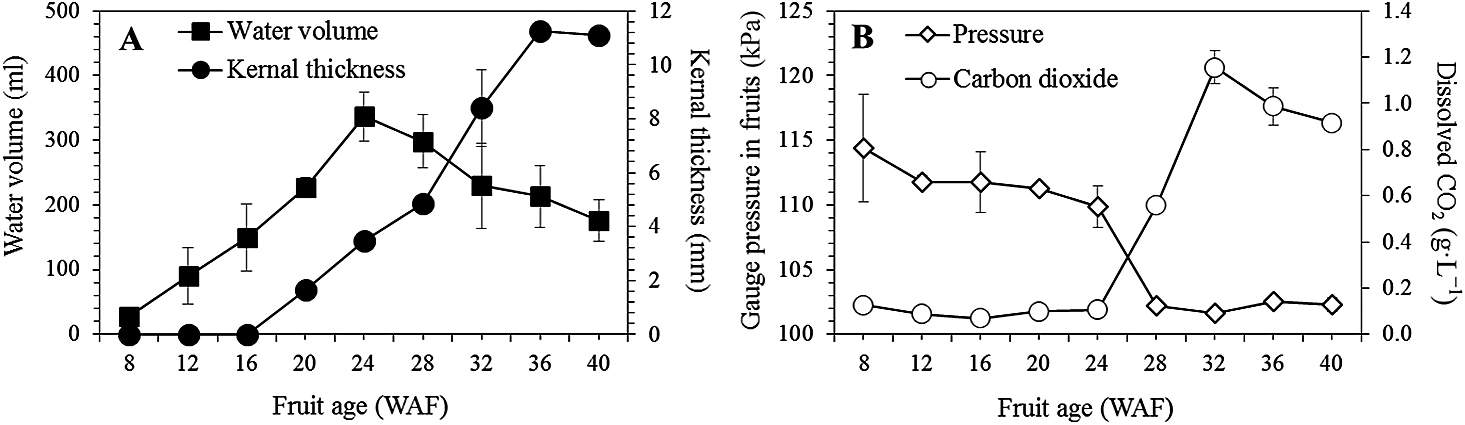
Coconut water volume and kernel thickness (A), and internal pressure and dissolved carbon dioxide concentration (B) in the coconut water during fruit growth. Bar on each data point indicates standard deviation, n = 9.
At harvest, the internal pressure of polished coconuts was 105 kPa, the same level as in the previous experiment; 24 h later, the pressure dropped below atmospheric pressure to 100.8 kPa. Dipping coconuts underwater for 24 h, one day after polishing, raised the internal pressure to the same level as at harvesting. The polished coconuts kept in the air had less than 1% cracking rate. For the coconuts kept underwater, the cracking rate increased to 18% after 24 h (data not shown).
(b) Effect of heatingAs the fruits were heated at 40°C, the temperature increased steadily over 4 h to 40°C. During this time, the internal pressure increased by 1 kPa after 1 h (Fig. 6A, B). At the end of the experiment, there was no significant change in the dissolved CO2 concentration in the coconut water. No shell cracking was found in the unheated fruit. Two out of the ten fruits in the heat treatment cracked. Hence, data from only eight fruits were used for statistical comparison. Water loss from the heated fruits was 2.8% after 4 h of heating.

Changes in water temperature (A), internal pressure (B), and CO2 concentration (C) of coconut water during heating from ambient temperature to 40°C. Bar on each data point indicates standard deviation, n = 8. *, ** Significantly different based on t-test, at P < 0.05 and 0.01, respectively.
After 24 h under atmospheric conditions, the internal pressure of the coconuts remained the same, similar to those stored under nitrogen (Fig. 7). However, in those kept under carbon dioxide, the pressure increased by about 0.5 kPa. The carbon dioxide concentration remained relatively stable for fruits stored under ambient conditions at 0.55 g·L−1. However, in those stored under nitrogen, the concentration decreased to 0.4 g·L−1, whereas in those stored under carbon dioxide, the concentration increased markedly to 1.25 g·L−1. At the end of the experiment, carbon dioxide concentrations were found to be 3.2, 1.2, and 100%, respectively, in the control, nitrogen, and carbon dioxide chambers. No shell cracking was observed in this experiment.

Internal pressure (A) and dissolved CO2 concentration (B) in young coconuts harvested at 28 ± 1 WAF and kept for 24 h under ambient, CO2, and N2 atmospheric conditions. Means with the same letter are not significantly different based on Tukey’s test, n = 6. P ≤ 0.05.
The internal pressure of polished coconuts at harvest was 101.7–103 kPa, which was higher than the atmospheric pressure (Fig. 8). The pressure dropped steadily to a level below atmospheric pressure (101.3 kPa) after 6 h. Shell cracking was observed only in fruits polished within 3 h after harvest (3.8–8.5%). No cracking was found when fruits were polished later. Carbon dioxide concentration determined just after harvest and 24 h later was similar at about 0.6 g·L−1.

Effect of delayed polishing time on the pressure inside the shell of young coconuts at 0–24 h after harvest at 28 ± 1 WAF (A), measured immediately after polishing (bar on each data point indicates standard deviation, n = 10), and on total cracking percentage, n = 20 (B).
This study revealed that shell cracking in polished young coconuts occurs when the shell is weak, in relationship to three factors: maturity stage, cracking site, and growing area. First, maturity stage was compared between cracked and non-cracked fruits. Most of the cracked fruits had a thinner shell and thinner kernel, indicating that the cracked fruits were younger, even though they were harvested at the same perceived age. This younger and thinner shell should also have less lignification (Nikhontha et al., 2019). Second, cracking site was observed principally on the biggest carpel, at the lower part, approximately half the distance from the fruit equator to the stylar end. The shell at this site is thinnest; this is likely due to the higher growth on this large carpel where the embryo develops compared to the other two carpels that are deprived of the embryo. Third, growing area was compared in fruits from two locations. Fruits from Damnoen Saduak, where a higher rate of cracking was found, were larger but had thinner shells than those from Ban Phaeo. The effect of fruit size is in concordance with other studies reporting that larger cherry or peach fruits are more prone to cracking than smaller fruits (Drogoudi and Pantelidis, 2022; Simon, 2006). The coconut growing areas are located next to each other but in different river basins. Damnoen Saduak belongs to the Mae Klong basin, whereas Ban Phaeo belongs to the Tha Chin basin. The soil in these two sites may have different minerals available for the coconut plants, resulting in different fruit growth and development.
The role of internal pressure on shell cracking was demonstrated in post-harvest manipulation of fruit pressure: 1) Dipping polished coconut in water or allowing water absorption into the fruit contributes to increasing the internal pressure up to 4 kPa and to increasing the shell cracking rate, similar to that reported in other fruits, e.g., Khanal et al. (2011); and 2) Heating polished coconuts by 10°C increased the internal pressure by 0.5 kPa. As the water expanded when the temperature increased, more cracking was observed. The role of temperature on coconut internal pressure is also partially shown. Data from 2019 to 2020 revealed a highly positive correlation between coconut water temperature and coconut internal pressure from Ban Phaeo. However, no relation was found in fruits from Damnoen Saduak nor in the 2013–2015 study.
Although high internal pressure in coconuts were found during 8–24 WAF, in agreement with the report by Scholander (1955), the role of internal pressure on fruit cracking incidence could not be demonstrated in the field studies. In the 2013–2015 seasons, relatively low pressure (101.6–102.7 kPa) and low incidence of cracking (mostly 0–3%) were observed throughout. When comparing the two growing locations in the 2019–2020 season, the internal pressure recorded in fruits from both sites were not different during most of the harvesting season. Conversely, when more cracking was observed in fruits from Damnoen Saduak (four out of eleven harvestings) than in Ban Phaeo, the internal pressure was lower than or did not differ to the fruits from Ban Phaeo.
When rainfall data was considered, correlation analysis showed that the accumulated rainfall 1, 3, 5, and 7 days before harvest was not related to the internal pressure of the fruit at harvest. This information suggests that the cracking of the shell did not depend on rainfall, as speculated by farmers.
The discrepancy between the field study and the manipulated pressure study may be because fruits in both studies were mainly at the 28 WAF maturity stage when the pressure became relatively low and the shell had been relatively hardened. Hence, the relation between internal pressure and shell cracking could not be established. In contrast, in the postharvest manipulated study, extra pressure was introduced into the fruit by dipping the fruit in water or by heating. Hence, there was enough pressure to cause cracking.
Our results do not support the farmers’ experiences that shell cracking was more common in the rainy season. This difference could be because the coconut fruit used in this study were collected at approximately 28 WAF (suitable for export), having sweet coconut water but a relatively thick and tough kernel. However, fruits harvested and sold by farmers in local markets, where consumers prefer to consume the coconut water together with the soft kernel, were approximately two to three weeks younger. These younger fruits are more likely to have higher internal pressure with weaker shells. At this earlier stage, the pressure inside may depend on rainfall and the water availability in the soil. This point should be further studied in the future.
The possibility that carbon dioxide accumulated in the coconut water contributes to the internal pressure was minor in this study. Since high internal pressure was never identified when high concentrations of carbon dioxide were recorded during fruit development, times of the year, or growing locations, the only time carbon dioxide would cause an increase in internal pressure large enough to cause shell cracking is when the fruits were stored under an unrealistic carbon dioxide-only atmosphere.
The primary source of carbon dioxide dissolved in coconut water is likely fatty acid synthesis; this process releases carbon dioxide as an end-product (Ohlrogge et al., 2015). High carbon dioxide accumulation, at 28 WAF and after, coincides with a report that oil accumulation rate in Malayan Yellow Dwarf coconuts was fastest at seven months of development (Rachel et al., 2010). As the kernel accumulates lipid, carbon dioxide is released into the coconut water.
Nevertheless, high carbon dioxide found in fruits over 28 WAF may account for the fizzy taste of coconut water and the phenomenon whereby gas and liquid flush out vigorously from the fruit after the shell is struck open or after piercing the soft eye to draw water for consumption. This is because such action is comparable to shaking a bottle of soda. The dissolved carbon dioxide changes from liquid to gas phase. Once an opening occurs, the gas expands immediately and gushes out of the bottle. However, shaking did increase the pressure inside, as shown in a study on carbon dioxide and the pressure in Champagne bottles (Vreme et al., 2015).
For practical purposes, when a high shell cracking rate and high internal pressure are encountered, delaying harvesting for about a week is recommended to allow further development of the shell, to become stronger and better withstand the pressure. Trimming and polishing after harvest should also be delayed to enable partial dehydration of the coconut by which internal pressure is reduced, hence preventing cracking. This process may take up to one day if the initial pressure is very high. Fruits should be left in an open space with good ventilation to achieve rapid water loss. The husk may be partially removed, leaving the fruit for some time to speed up water loss before polishing. To avoid disrupting the production line in the packing house, dry or warm air may be arranged to accelerate the dehydration time.
During the preparation of trimmed or polished young coconuts, dipping the fruits in sodium metabisulfite solution is a common practice to prevent browning and mold growth on the surface (Mohpraman and Siriphanich, 2012). From our observations, dipping fruits in water raised the internal pressure by 1 kPa within 6 minutes (Supplement Fig. 1). Hence, the dipping period should also be brief. This is not only to prevent over-absorption of water into the fruit that would otherwise increase the internal pressure, leading to shell cracking, but also to avoid exceeding the limit of chemical residues.
In conclusion, shell cracking of polished young coconuts was found to be related to the strength of the shell, which depends on fruit maturity and growing location. Fruits with thinner shells are more susceptible to cracking. The force that causes cracking is the internal pressure, which is high in younger fruits and becomes lower to slightly above atmospheric pressure seven months after full bloom. We also found that fruit internal pressure is not related to rainfall. Carbon dioxide accumulated in the coconut water was only a minor factor that contributed to the high pressure inside the fruit. To reduce shell cracking incidence, it is recommended to delay harvesting to allow time for the shell to further harden, as well as delay the trimming and polishing processes to allow water loss from the fruit and reduce the pressure inside.
This work was supported by the Center for Advanced Studies for Agriculture and Food, Institute for Advanced Studies, Kasetsart University, Under the Higher Education Research Promotion and National Research University Project of the Higher Education Commission Ministry of Education, Thailand (CASAF PM077); Research and Researchers for Industries Project, Thailand Science Research and Innovation (MSD5710008); K-Fresh & All Coconut Group; Postharvest Technology Innovation Center, Thailand. The authors thank Anak Pakcharoen and Wannipa Suksombat for their untiring help with collecting data.