2023 年 92 巻 2 号 p. 151-161
2023 年 92 巻 2 号 p. 151-161
Artificial pollination is essential in pears (Pyrus spp.) for stable fruit set and the production of high-quality fruit. Low temperatures in the early spring before bud flowering can damage pear pollen and pose a special risk to pollen production for artificial pollination. In this study, we sought to identify, in pears, the flower bud stage most susceptible to low-temperature pollen damage, observe anther development abnormalities, compare pollen sensitivities among seven pear cultivars, and evaluate the pollen cold injury inhibition efficacy of a coffee extract treatment for flower buds. The most cold-sensitive flower bud stage for the Japanese pear ‘Kosui’ pollen was the scale-separation stage, with pollen in that stage ranging from the pollen mother cell to the tetrad stage. In the ‘Kosui’ flower bud treated at −3°C over 10 h, the typical cold-induced anther development abnormalities were tapetum hypertrophy, cell debris, and anther locule shrinkage. Flower buds at the scale-separation stage of seven pear cultivars were treated with low temperatures in a model based on daily temperature changes during the winter season in Saitama, Japan, to reach daily minimum temperatures of 0, −1, −2, −3, −4, −5, and −6°C. The pollen germination rates of ‘Hosui’ were unaffected by low-temperature treatments between 0 and −6°C. Overall ‘Hosui’ showed the least reduction in pollen germination rate than the other cultivars evaluated. Our results strongly suggest that ‘Hosui’ is the most tolerant to low temperatures among the tested cultivars. In the laboratory environment, the application of a coffee extract before cold treatment delayed flower bud freezing and preserved pollen germination. These results are helpful in selecting varieties and treatments for the prevention of cold injury in pear pollen production.
In pears (Pyrus spp.) that are self-incompatible, artificial pollination is essential for stable fruit set and high-quality fruit production. Numbers of natural pollinators such as Hymenoptera have fallen in recent years (Sanchez-Bayo and Wyckhuys, 2019), but pear flowers have lower honey and sugar content than other fruit trees, and are generally less attractive to pollinators (Quinet et al., 2016b). Pollinator the activity also depends on the weather (Clarke and Robert, 2018), with the gap in bloom dates between cultivars limiting insect pollen transport (Quinet et al., 2016a). Due to these factors, artificial pollination is essential to stabilize fruit set and improve fruit quality. In apples (Malus domestica Borkh.) artificial pollination is reported to produce higher-quality fruits than natural pollination (Samnegard et al., 2019).
More than half of Japanese pear producers in Japan use imported pollen for artificial pollination (Maeshima et al., 2018a), but imported pollen may include pathogens such as Erwinia amylovora, the cause of fire blight (Kim et al., 2021), which has not yet been found in Japan. Ideally, domestic pollen should be used in Japanese pear production. Pear producers who do not use imported pollen expend great effort and resources to collect pollen for artificial pollination (Maeshima et al., 2018b). For these reasons, it is necessary to establish an efficient domestic pollen-production system.
One of the greatest risks for pollen production is low temperature damage to pollen in the early spring before bud flowering. Indeed, low temperature pollen damage impacts pollen formation in a wide variety of crops. Low temperatures before blooming are shown to reduce pollen fertility and quantities in rice (Oryza sativa L.) (Satake, 1991; Yamamori et al., 2021), wheat (Triticum aestivum L.) (Barton et al., 2014), maize (Zea mays L.) (Tranel et al., 2009), soybean (Glycine max L.) (Ohnishi et al., 2010), chickpea (Cicer arietinum L.) (Clarke and Siddique, 2004), sorghum (Sorghum bicolor Moench) (Wood et al., 2006) and pepper (Capsicuum annuum L.) (Shaked et al., 2004). The pollen development stages most sensitive to cold differ according to species: the young microspore stage (tetrad to early microspore stage) in rice (Satake, 1976, 1991; Satake and Hayase, 1970), the dyad stage during microsporogenesis in wheat (Barton et al., 2014), the pre-tetrad to tetrad stage in soybeans (Ohnishi et al., 2010), and meiosis to the tetrad stage in chickpeas (Clarke and Siddique, 2004). Cold-induced pollen sterility in rice is associated with abnormal formation of the tapetum and anther locules (Nishiyama, 1970; Oda et al., 2010; Yamamori et al., 2021). Cultivars within a species can also differ in the specifics of their pollen cold sensitivities, as shown among the 13 rice cultivars studied by Yamamori et al. (2021).
In Japanese pears (P. pyrifolia Nakai), there are many studies on the influence of low temperatures on female organs (Oya, 2012; Sakuma et al., 2013; Sato, 1982; Sekozawa et al., 2003), but few on the effect upon pollen. Previously, it was reported that the tetrad to early microspore stage of pollen development was most sensitive to low temperature in Japanese pears (Hirata, 2000), but low-temperature sensitivity among cultivars and temperatures at which damage occurs remain unclear. Furthermore, low temperatures are known to cause abnormal anther formation in other flowering plants, yet there is been no study on pear anthers.
Minimizing low temperature pollen damage is also important for pollen production. Wind machines, fuel burning, and overhead irrigation are options to prevent frost damage to fruit trees, but these measures are expensive (Smith, 2019). Chemical application is a less expensive method for frost protection (Smith, 2019). One possible chemical treatment can be derived from coffee refuse. These extracts have anti-ice-nucleation activity and can be isolated from food processing wastes, making production inexpensive (Kawahara et al., 2017). Caffeine, a major component of these extracts, binds to calcium oxalate in plants, which inhibits ice nucleation and freezing (Kawahara et al., 2017). Thus, coffee extract treatment may reduce cold injury, but its effect on pear pollen is unknown.
In this study, we first, sought to identify the bud flowering stage in Japanese pears most susceptible to cold-temperature pollen damage and, during this stage, observe the morphological effects of low temperature on anther development using ‘Kosui’ as a model cultivar. Second, we evaluated the cold sensitivity of pollen among seven pear cultivars using a Japanese climate temperature variation model. Finally, we examined the effectiveness of coffee extract treatment to inhibit cold injury to Japanese pear flower buds.
In January 2020, one-year-old branches of a 29-year-old Japanese pear ‘Kosui’ grafted onto Pyrus calleryana Decne and planted at the Kuki Branch of Saitama Agricultural Technology Research Center were collected. The branches were cut into 15-cm-long pieces, leaving the flower buds at the branch tips, and removing all other buds. Branches were inserted into floral foams and placed in containers filled with a solution of 1% sucrose, 10 ppm glutamic acid, and 0.03% aluminum sulfate and left to stand at 15°C for the continuation of flower development. Flower buds were removed at stage I to stage V (Fig. 1) and fixed in FAA solution (90: 5: 5 = 70% ethanol: formaldehyde: acetic acid). Three flowers from each flower bud at each flower bud stage were used for observation of pollen development.
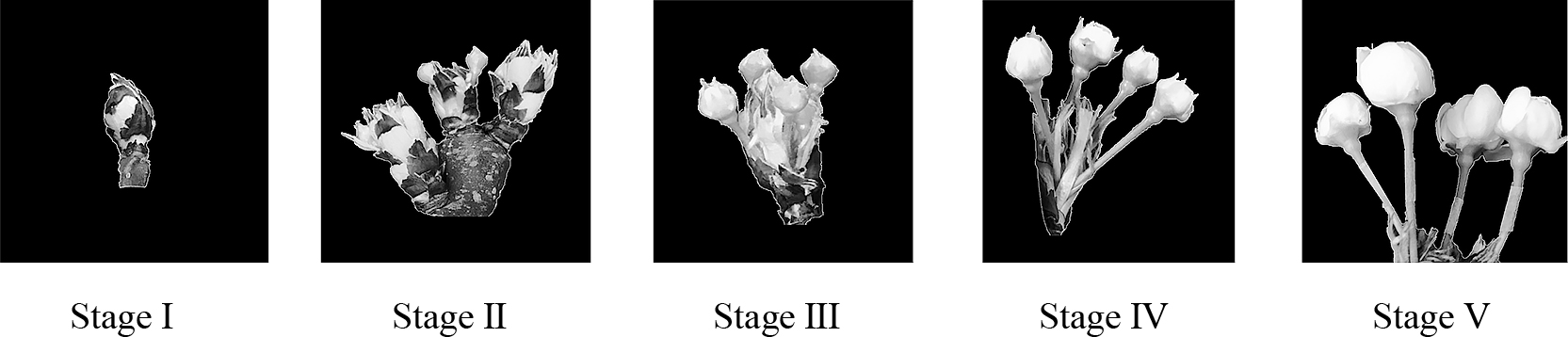
Flower bud stages of Japanese pear ‘Kosui’. Stage I, scale separation; Stage II, blossom bud; Stage III, before first white; Stage IV, full white; Stage V, balloon.
A typical anther was taken from each flower bud, and pollen grains from the anther were separated in a drop of water on a glass slide. After these were sealed with the cover glass, the pollen development stage was observed. Five fields of view per anther were selected randomly at 100× using a light microscope (BX53; OLYMPUS, Tokyo, Japan), and all anthers in the view fields were observed. More than 100 pollen grains for each replication were investigated. These were classified into eight stages as shown in Figure 3, based on the method of Hayashi and Tanabe (1991).
Effect of low temperature at different flower bud development stages on pollen density, pollen germination rate, and anther development 1. Pollen densityThe experiment was conducted in 2020. Three one-bud branches of ‘Kosui’ were used for each stage of low-temperature exposure to observe pollen density. Branches at each flower bud stage I to V were removed from floral forms, sprayed with water, wrapped in a plastic bag, and covered with newspaper. Then, the branches were subjected to low-temperature treatments using an incubator (LTE-510; EYELA, Tokyo, Japan). The low-temperature treatments were carried out by holding the incubator at 15°C for 5 min, cooling to 0°C or −3°C over 6 h, holding for 10 h, and then raising the temperature to 10°C over 4 h. Branches treated by low temperature at stages I to IV were once again inserted into floral foams and kept at 15°C until they reached stage V and were then collected. Cold-treated flower buds at stage V were collected immediately after low-temperature treatment. Collected flower buds were fixed with FAA solution.
A representative anther was taken from each flower bud, and paraffin sections were prepared according to Miura et al. (2011). Fixed anthers were dehydrated by soaking in a decreasing series of ethanol dilutions (50, 70, 85, 95, and 99.5%) for 2 h each. Then, the anthers were soaked in an ethanol-butanol series (3:1, 2:2, 1:3, 0:4) for 2 h each, replaced with paraffin, and embedded into paraffin blocks. The paraffin blocks were trimmed and sliced into 10-μm sections that were placed on glass slides and deparaffinized with xylene. After staining with Delafield’s hematoxylin and eosin, the samples were then sealed with Eukitt solution. Anther sections were observed at 100× by light microscope, with anther pollen density classified into five stages according to Figure 2 and assigned a pollen density index (PDI).

Pollen density index (PDI) in Japanese pear ‘Kosui’. Paraffin sections of anthers at flower bud stage V were prepared and classified into five stages of pollen density in the anther section. Scale bars = 100 μm.
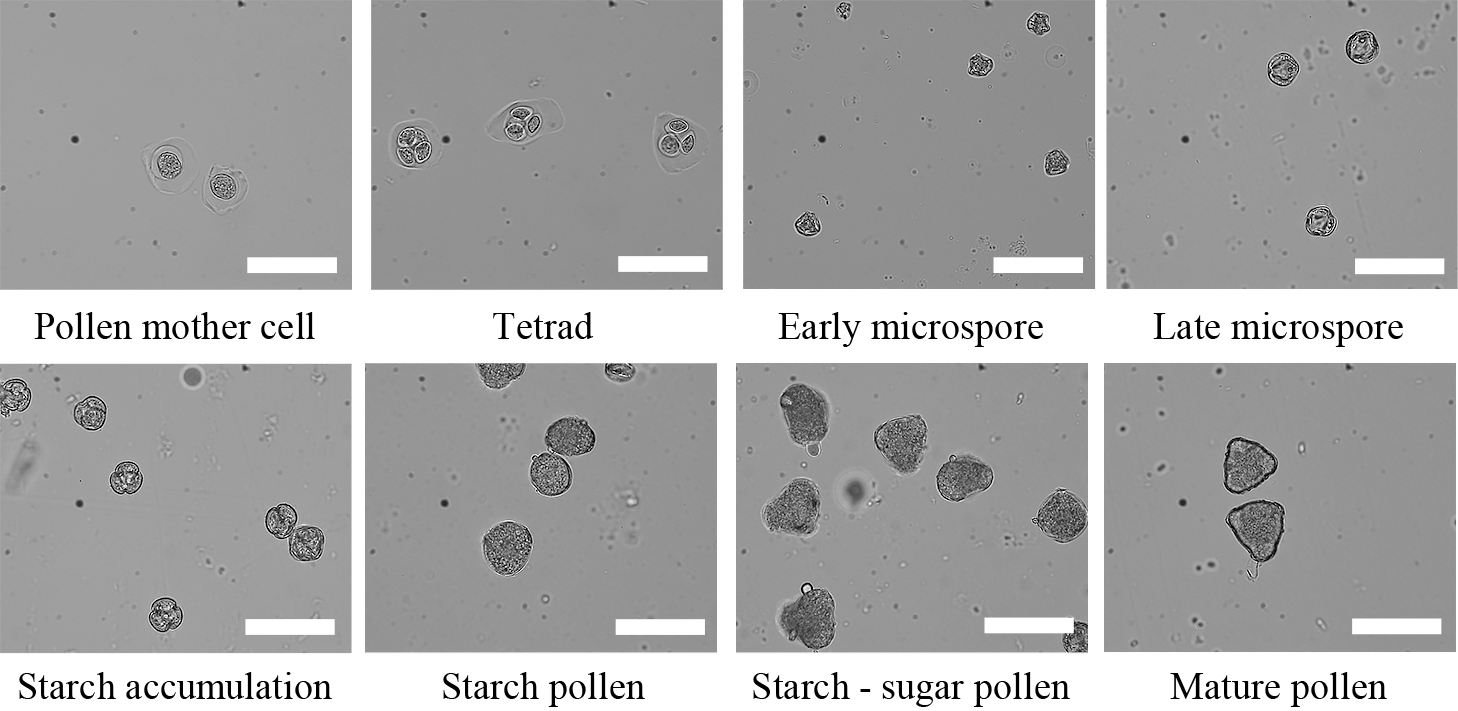
Pollen stages in Japanese pear ‘Kosui’. Scale bars = 50 μm.
The experiments were conducted in 2021 and 2022. Five one-bud branches of ‘Kosui’ were used for each treatment in both years to investigate pollen germination rate. Branches were treated at each flower bud stage, I to V, using the incubator sequence described in section 1; then all anthers were harvested from each flower bud upon reaching stage V. A control without the low-temperature treatment was established, from which all anthers were taken from a stage V flower bud. Anthers were placed in an anther-opening machine (M-160; MITSUWA, Niigata, Japan) at 25°C for one day to induce anthesis. After anthesis, anthers were frozen at −20°C for storage. The pollen germination rate was investigated after the frozen anthers had rested at room temperature for 2 h. Pollen grains were collected from anthers with a cotton swab, spread on a medium (10% sucrose and 1% agar), and incubated at 25°C for 2 h. From each flower bud, 150 pollen grains were investigated at 30× by a stereomicroscope (LZ-LED-T KENIS, Osaka, Japan), and the pollen germination rate was calculated by dividing the number of germinated pollens by 150.
3. Process of anther developmentThe experiment was conducted in 2020. Three one-bud branches of ‘Kosui’ were used for each treatment to observe anther development. Branches at flower bud stage I were treated at low temperature as described in section 1; flower buds reaching stage II to V were collected, respectively, and fixed with FAA solution. A typical anther was taken from each flower bud and prepared in paraffin sections as described in section 1. The development process of anthers was observed at 200× by light microscope.
Effect of low temperature on pollen germination rate among seven pear cultivarsThe experiments were conducted in 2021 and 2022. In January of both years, one-year-old branches were collected from the following Japanese pear trees: a 19-year-old (2021) and 20-year-old (2022) ‘Akizuki’, a 50-year-old (2021) and 51-year-old (2022) ‘Hosui’, a 30-year-old (2021) and a 31-year-old (2022) ‘Kosui’, a 55-year-old (2021) and a 56-year-old (2022) ‘Matsushima’, an 18-year-old (2021) and a 19-year-old (2022) ‘Saigyoku’, a 10-year-old (2021) and an 11-year-old (2022) ‘Yasato’. In addition, samples from these a 20-year-old (2021) and a 21-year-old (2022) Taiwanese pear (P. pyrifolia Nakai) ‘Yokoyamanashi’. The latter was planted in the fields of the Institute of Fruit Tree and Tea Science, National Agriculture and Food Research Organization while the others were planted at the Kuki Branch of the Saitama Agricultural Technology Research Center. ‘Yasato’ and ‘Yokoyamanashi’ were grafted onto compatible rootstock, and others were grafted onto P. calleryana Decne. Five one-bud branches of these cultivars at flower bud stage I in both years were used for each low-temperature treatment for every cultivar.
For the low-temperature treatment, we created a field model that closely resembled field conditions. The field model was based on Sakuma et al. (2013) and modified to reflect spring temperature changes in Kuki City, Saitama Prefecture. The average hourly temperature in Kuki City, from February 1 to March 20, 2020, reached its maximum at 3:00 p.m., decreased over 15 h, and reached its minimum at 6:00 a.m. the next day. Therefore, the 12 h it took to cool from the maximum temperature to the minimum temperature in the experiment by Sakuma et al. (2013) was changed to 15 h here. In other words, the treatments were carried out by holding the temperature at 15°C for 5 min, cooling the incubator to 0, −1, −2, −3, −4, −5, or −6°C over 15 h, holding at that minimum temperature for 1 h, then raising the temperature to 10°C over 4 h. In addition, a control of each variety without a low-temperature treatment was established. After the low-temperature treatments, all the anthers at the first flower from each flower bud that reached stage V and underwent anthesis were collected and stored similarly as described in section 2.
Before the pollen germination rate investigation, the anthers were kept at room temperature for 2 h and mixed with 500 μL of acetone in a 2 mL microcentrifuge tube for 30 sec. After drying the acetone, the pollen germination rate was investigated as described in section 2. In addition, we set the value calculated as the pollen germination rate of the control (no low-temperature treatment) as 100, against which other obtained values were scaled (this is as follows: control as 100). Five replications were performed in 2021 and 2022.
Effect of coffee extract treatment on freezing and pollen germination rate of flower budsThe experiments were conducted in 2021 and 2022. To investigate the freezing point and pollen germination rate, five one-bud branches of ‘Kosui’ were used for each treatment, respectively. The freezing point was investigated in 2021, and the pollen germination rate was investigated in 2021 and 2022. Coffee extract (Frostbuster, KUREi, Osaka, Japan) at 2 ppm was sprayed on the surface of flower buds and branches at stage I. In the control, water was sprayed. After treatments, branches were inserted into floral foam and kept at 15°C for 20 h to allow the treatment to dry. After that, low-temperature treatments were carried out at −3°C.
The freezing point of flower buds was investigated by differential thermal analysis (DTA). In this experiment, a thermometer (RT-31S; ESPEC, Osaka, Japan) was attached to the surface of flower buds in each treatment to measure the temperature every 10 sec. The freezing point of flower buds was determined based on Meng et al. (2007) (Fig. 4); namely, we investigated the time at −3°C required for the flower buds to freeze.

Variation curve of flower bud temperature. A thermometer was attached to the surface of a flower bud to measure the temperature every 10 sec at −3°C. The freezing point was identified as the point at which the endotherm and exotherm were balanced.
To investigate the pollen germination rate, low-temperature treatments were carried out at −3°C for 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 h after chemical treatment at flower bud stage I. After low-temperature treatment, all the anthers of the first flower buds to reach stage V and undergo anthesis were collected and stored as described in section 2. Pollen was separated from anthers using acetone, and the pollen germination rate was investigated as described in section 2.
At flower bud stage I, associated with the pollen stages from the pollen mother cell to the early microspore, the pollen mother cell stage accounted for 71.0%, followed by the tetrad stage at 22.4%. Flower bud stage II contained pollen stages from the early microspore to starch accumulation, with early and late microspore stages comprising 38.6% and 56.4% of the pollen, respectively. Flower bud stage III contained pollen stages from the early microspore to the starch pollen, with late microspore and starch accumulation stages comprising 48.3% and 47.8% of the pollen, respectively. At flower bud stage IV, when pollen ranged from the late microspore to the mature pollen stage, the starch pollen stage comprised 60.2%. Flower bud stage V contained pollen stages from starch accumulation to the mature pollen, with about the same abundance in the starch pollen stage as in flower bud stage IV. But at flower bud stage V, the starch-sugar pollen stage and the mature pollen stage were higher and the starch pollen stage was lower than in flower bud stage IV (Table 1).

Pollen stages in each flower bud stage for Japanese pear ‘Kosui’ (2020). Three flowers for each flower bud stage were used for pollen stage observation, with more than 100 pollen grains per one flower (n = 3).
Pollen density in anthers was significantly reduced after −3°C treatment at flower bud stage I compared to treatments at other flower bud stages. No significant differences were observed among the other stage treatments (Table 2). The −3°C treatment at flower bud stage I also significantly reduced the pollen germination rate compared to the control for both years. No significant differences were observed for the other stage treatments (Fig. 5A and B). In the 0°C treatment, all anthers developed normally (Fig. 6 A, D, F, and H): the tapetum and microspores were observed in flower bud stage II (Fig. 6 A), the tapetum was smaller in flower bud stage III (Fig. 6 D), and normal pollen grains were formed in flower bud stage V (Fig. 6 H). On the other hand, at the −3°C treatment, tapetum hypertrophy and degraded cells were observed in flower bud stage II (Fig. 6 B and C), cell debris was observed in flower bud stage III (Fig. 6 E), and anthers were smaller than controls in flower bud stages IV and V (Fig. 6 G and I).
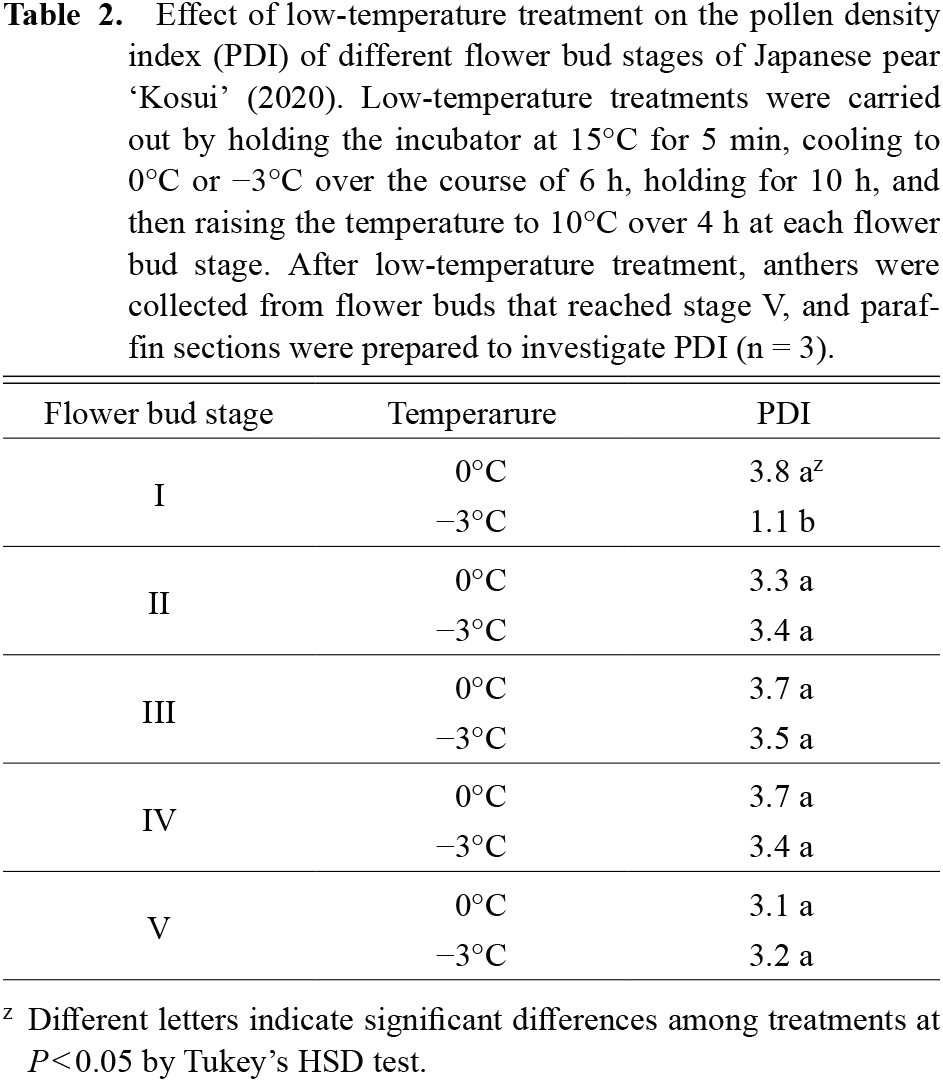
Effect of low-temperature treatment on the pollen density index (PDI) of different flower bud stages of Japanese pear ‘Kosui’ (2020). Low-temperature treatments were carried out by holding the incubator at 15°C for 5 min, cooling to 0°C or −3°C over the course of 6 h, holding for 10 h, and then raising the temperature to 10°C over 4 h at each flower bud stage. After low-temperature treatment, anthers were collected from flower buds that reached stage V, and paraffin sections were prepared to investigate PDI (n = 3).

Effect of low-temperature treatment at different flower bud stages on pollen germination rate in Japanese pear ‘Kosui’ (2021 and 2022). Low-temperature treatments were carried out by holding at 15°C for 5 min, cooling to 0° C or −3°C over 6 h, holding for 10 h, and then raising the temperature to 10°C over 4 h at each flower bud stage. After low-temperature treatment, pollen grains were collected from flower buds that reached stage V to investigate the pollen germination rate. Values indicate means ± SEs (n = 5). * and NS indicate significantly lower values than control at P < 0.05 and no significant difference from the control for each year, respectively, by Steel’s test.
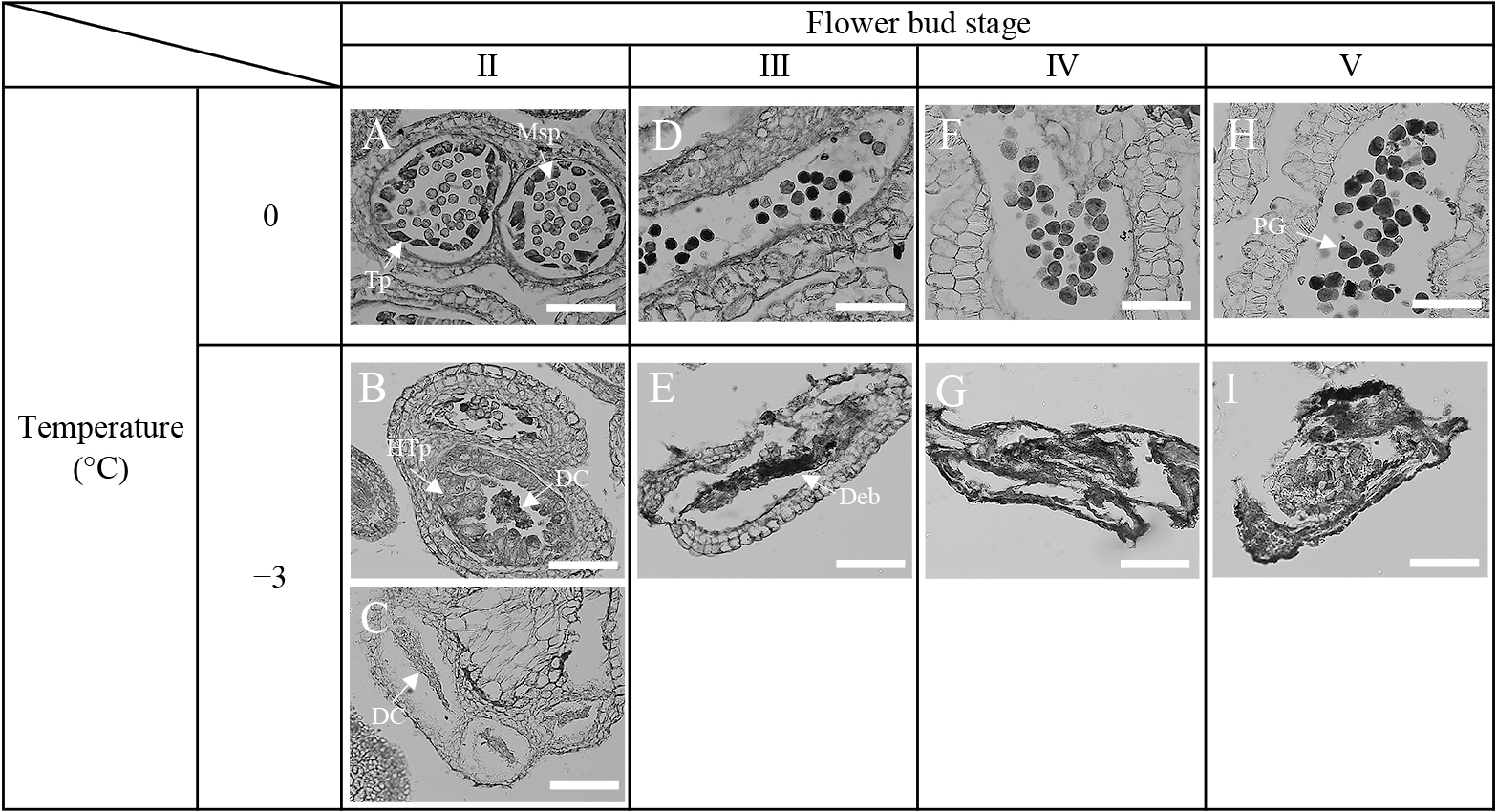
Comparison of anther development in low-temperature treatments at 0°C and −3°C in Japanese pear ‘Kosui’ (2021). Low-temperature treatments were carried out by holding the incubator at 15°C for 5 min, cooling to 0°C or −3°C over 6 h, holding for 10 h, and then raising the temperature to 10°C over 4 h at flower bud stage I. After the low-temperature treatment, anthers were collected from flower buds at stage II to V, and paraffin sections were prepared to observe anther development (n = 3). Msp, microspore; Tp, tapetum; HTp, hypertrophic tapetum; DC, degraded cell; Deb, debris; PG, pollen grain. Scale bars = 100 μm.
First, the pollen germination rates were compared among low-temperature treatments in each cultivar. The pollen germination rates of ‘Akizuki’ at −3, −4, −5, and −6°C in 2021 and at −4 and −6°C in 2022 were significantly lower than that of the untreated control. The pollen germination rates of ‘Kosui’ at −4, −5, and −6°C in 2021, −6°C in 2022 were significantly lower than that of the control. The pollen germination rates of ‘Matsushima’ at −4, −5, and −6°C in 2021 and 2022 were significantly lower than that of the control. The pollen germination rates of ‘Saigyoku’ at −5 and −6°C in 2021 and at −4 and −6°C in 2022 were significantly lower than that of the control. The pollen germination rates of ‘Yokoyamanashi’ at −6°C in 2021 were significantly lower than that of the control. The pollen germination rates of ‘Yasato’ and ‘Hosui’ in both years were not significantly different between temperature treatments and control. Next, the pollen germination rates were compared among the cultivars at each low-temperature treatment. The pollen germination rates at −1°C of ‘Akizuki’, ‘Matsushima’ and ‘Yokoyamanashi’ in 2021 were significantly higher than that of ‘Kosui’ (Table 3).
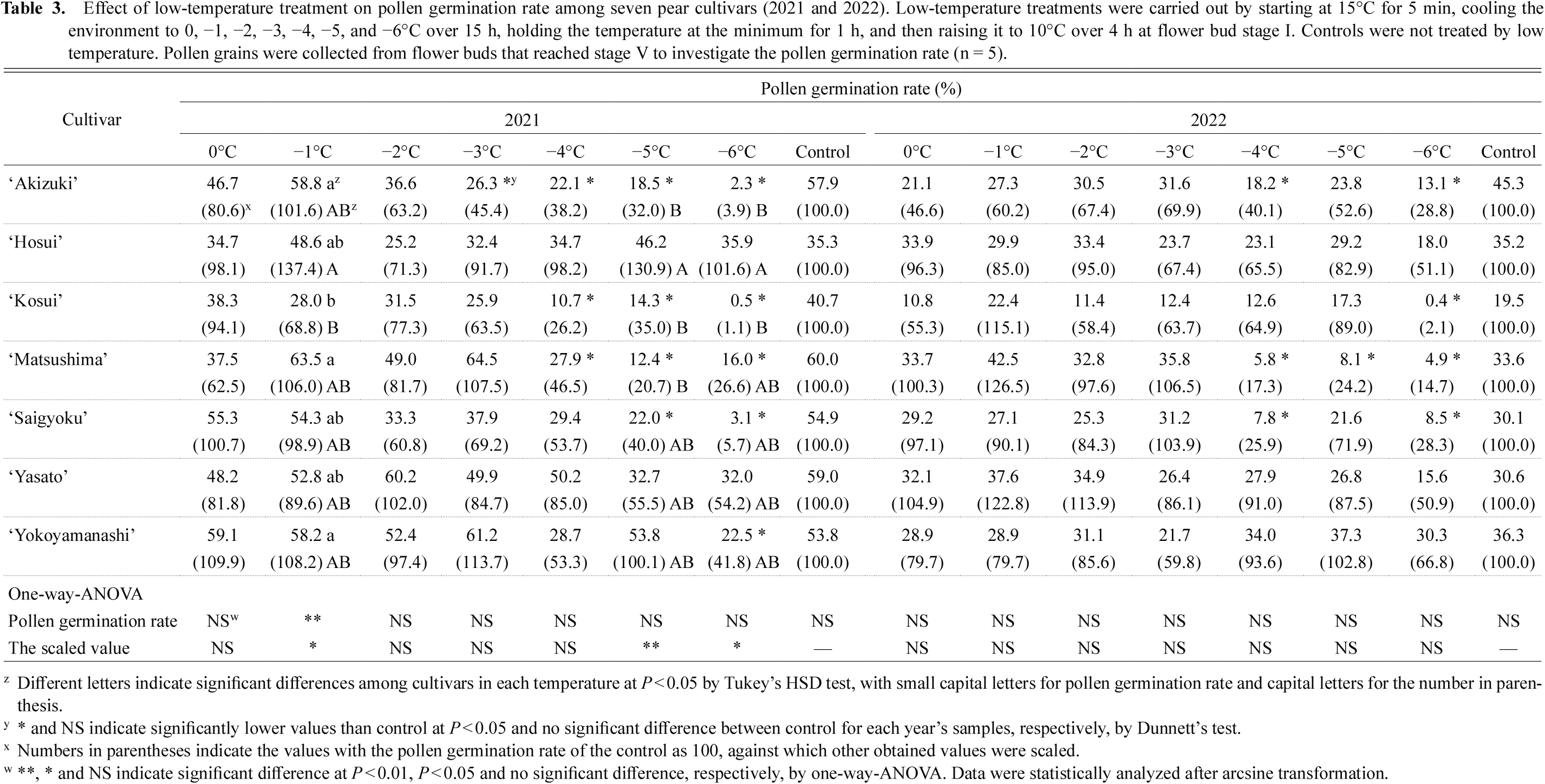
Effect of low-temperature treatment on pollen germination rate among seven pear cultivars (2021 and 2022). Low-temperature treatments were carried out by starting at 15°C for 5 min, cooling the environment to 0, −1, −2, −3, −4, −5, and −6°C over 15 h, holding the temperature at the minimum for 1 h, and then raising it to 10°C over 4 h at flower bud stage I. Controls were not treated by low temperature. Pollen grains were collected from flower buds that reached stage V to investigate the pollen germination rate (n = 5).
In 2021, the scaled value against control as 100 at −1°C was significantly higher for ‘Hosui’ than for ‘Kosui’. The scaled value against control as 100 at −5°C was significantly higher for ‘Hosui’ than for ‘Akizuki’, ‘Kosui’ and ‘Matsushima’. The scaled value against control as 100 at −6°C was significantly higher for ‘Hosui’ than for ‘Akizuki’ and ‘Kosui’ (Table 3).
Effect of coffee extract treatment on flower bud freezing point and pollen germination rateThe time required for flower buds to freeze was about 8 h with coffee extract treatment compared to about 3 h in controls (Fig. 7). In terms of the effect of coffee extract treatment on pollen germination rate, significant differences were found in the factor of treatment in both 2021 and 2022; namely, the pollen germination rates after coffee treatment were higher than controls in both years (Table 4).
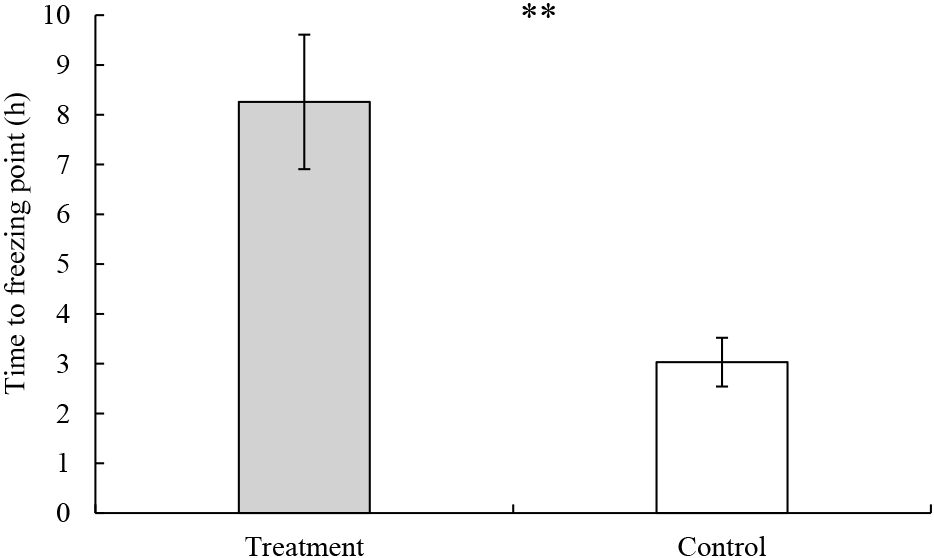
Effect of coffee extract on time to reach the freezing point of flower buds in Japanese pear ‘Kosui’ (2021). Treatments and controls were respectively sprayed with coffee extract and water at flower bud stage I and allowed to dry completely before low-temperature treatment at −3°C. The temperature of the flower bud surface was measured under −3°C to investigate time to the freezing point. Values indicate means ± SEs (n = 5). ** indicates significant difference at P < 0.01 by t-test.
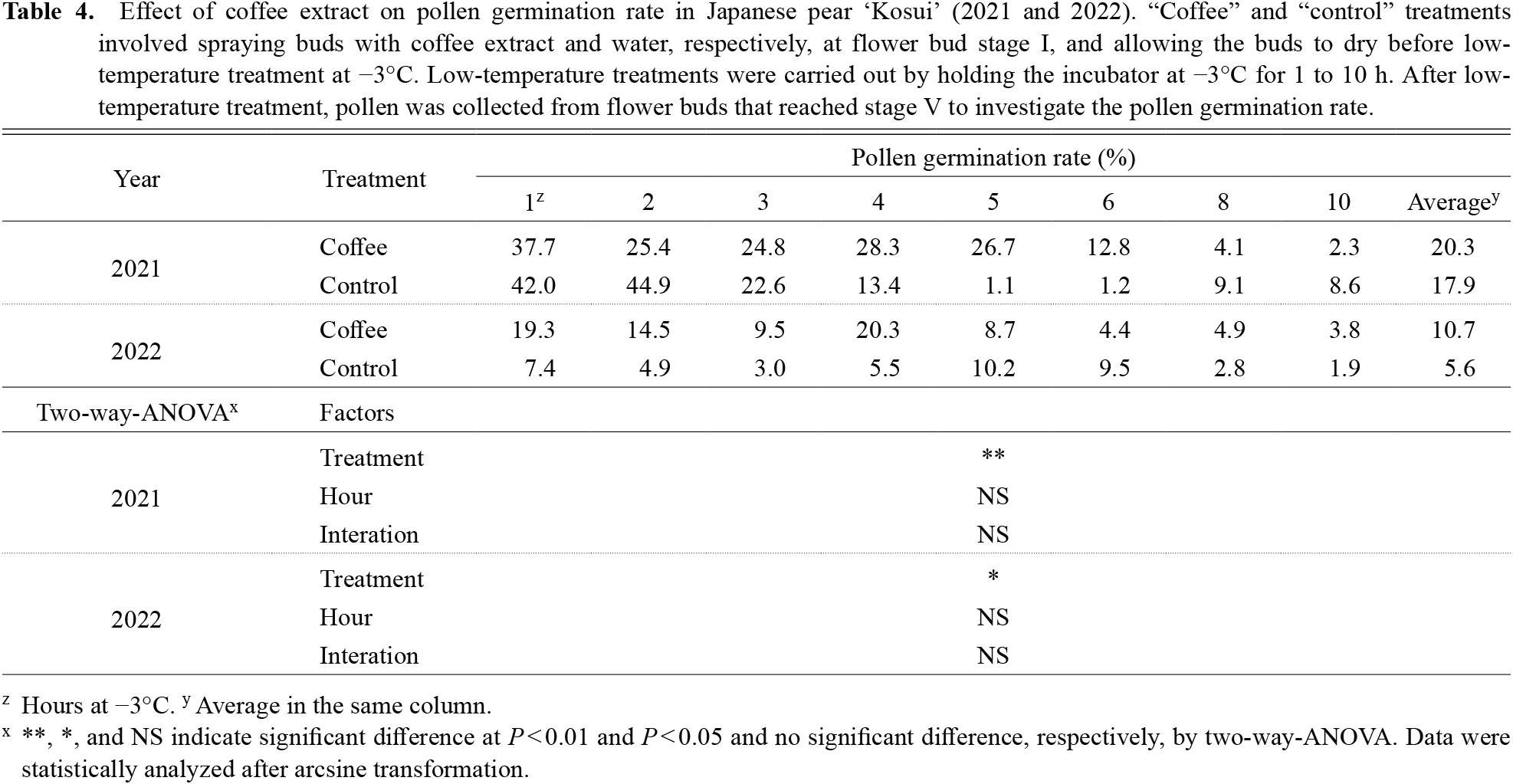
Effect of coffee extract on pollen germination rate in Japanese pear ‘Kosui’ (2021 and 2022). “Coffee” and “control” treatments involved spraying buds with coffee extract and water, respectively, at flower bud stage I, and allowing the buds to dry before low-temperature treatment at −3°C. Low-temperature treatments were carried out by holding the incubator at −3°C for 1 to 10 h. After low-temperature treatment, pollen was collected from flower buds that reached stage V to investigate the pollen germination rate.
In the Japanese pear ‘Kosui’, the pollen density and pollen germination rate were significantly decreased by 10-h low-temperature treatment at −3°C in flower bud stage I but not at other stages. Therefore, flower bud stage I is considered the stage most sensitive to low temperature for pollen formation and development. In contrast to these results for male organs, reports have shown that the more advanced the flower bud stage of Japanese pear, the more sensitive the female organs are to low temperatures (Oya, 2012; Sakuma et al., 2013). Browning of the female tissue due to low-temperature exposure has been observed (Oya, 2012; Sakuma et al., 2013; Sato, 1982). Sugar accumulation inhibits intracellular freezing and prevents frost injury in the Japanese pear flower (Sekozawa et al., 2003). The sugar content of a Japanese pear bud decreases from winter to spring (Sekozawa, 2004) whereas in the pollen, starch is turned into sugar, and sugar accumulates in the pollen grain as it matures (Hayashi and Tanabe, 1991). A higher free sugar content in pear flowers reduced low-temperature injury (Lee et al., 2023). Thus, differences in the timing of increased sensitivity to low temperatures in male and female organs may be related to differences in the timing of the increase in sugar content.
In flower bud stage I, the pollen stages of pollen mother cell, tetrad and early microspore were observed, with pollen mother cell and tetrad being the main stages. This suggests that the effect of low temperature on pollen formation and development depends on the pollen developmental stage, with the most cold-sensitive stage in the Japanese pear ‘Kosui’ being the pollen mother cell to tetrad stage. Research in other crops has shown that the pollen stages most sensitive to low temperature in soybeans and chickpeas are the pre-tetrad to tetrad stage (Ohnishi et al., 2010) and the meiosis to the tetrad stage (Clarke and Siddique, 2004), respectively. These reports agree with our results. On the other hand, Hirata (2000) previously reported that the pollen stages most sensitive to low temperature in Japanese pear were the tetrad to early microspore stages, slightly later than our results. The disparity may stem from differences in cultivar.
In our study, various anther development abnormalities were observed in ‘Kosui’ flower buds treated at −3°C for 10 h in flower bud stage I, including tapetum hypertrophy, cell debris, and locule shrink. Low-temperature-induced tapetum hypertrophy and abnormal pollen morphology were also associated with male sterility in the rice cultivars ‘Nourin 20’ (Nishiyama, 1970) and ‘Sasanishiki’ (Oda et al., 2010). We observed abnormalities reflecting to these reports, suggesting that low-temperature-induced anther formation abnormality is one factor reducing pollen density and pollen germination rates. Not surprisingly, the pollen germination of ‘Kosui’ treated with −3°C for 1 h in the field model was higher than that treated with −3°C for 10 h. Low temperatures like −3°C for 10 h are uncommon in areas where pears are grown in Japan. Therefore, the severe pollen abnormalities observed at −3°C for 10 h may not necessarily occur in the field.
Differences in low-temperature sensitivity among cultivarsThe degree of reduction in pollen germination by low-temperature exposure in flower bud stage I differed among the seven cultivars. This result suggests that the effect of low temperature on pollen development depends on the pear variety. Pollen germination rates of ‘Hosui’ were unaffected by low temperatures either year. The scaled value, against the ‘Hosui’ control as 100, were higher than those of the other cultivars in 2021. Therefore, it is suggested that ‘Hosui’ pollen is the most tolerant to low temperatures at flower bud stage I among the tested varieties. Plant hormones are among the factors that cause varietal differences in pollen sensitivity to low temperature. In both rice anthers (Ji et al., 2011; Oliver et al., 2007) and wheat spikelets (Zhang et al., 2019), low-temperature treatment increased ABA content in cultivars with cold-sensitive pollen compared to those with cold-tolerant pollen. ABA is known to negatively regulate the expression of cell wall invertase genes in rice anthers (Ji et al., 2011). Decreased invertase activity in rice anthers is shown to reduce pollen fertility by interfering with hexose production and decreasing pollen starch accumulation (Oliver et al., 2005). Gibberellic acid (GA) content in wheat spikelets was significantly reduced by low temperature in a pollen cold-sensitive cultivar compared to a cold-tolerant cultivar (Zhang et al., 2019). In rice anthers, reduction in GA content and accumulation of its negative regulator, DELLA protein, by low temperature results in abnormal tapetum hypertrophy (Sakata et al., 2014). Therefore, different effects in different cultivars of low temperature on ABA and GA may be one of the reasons for the differences in cold sensitivity of pollen among cultivars. The effects of low temperature on these plant hormones should be investigated in pears.
In our experiment, pollen germination of all cultivars was evaluated at flower bud stage I, which was the most sensitive to low temperature in ‘Kosui’. However, the pollen stage in flower bud stage I may differ among cultivars, including ‘Kosui’. Effects of low temperature on cultivars other than ‘Kosui’ need further investigation. Pear cultivars that reach flower bud stage I earlier are at higher risk of low temperature exposure. In Saitama Prefecture, ‘Yokoyamanashi’ reached flower bud stage I as early as mid-February (unpublished data). Therefore, when selecting cultivars for use, it is necessary for producers to consider not only the cold tolerance of the cultivars but also when the cultivars reach flower bud stage I in the field.
Effect of coffee extractsTreatment with coffee extract extended the time required to freeze flower buds as measured by DTA and effectively maintained the pollen germination rate under low temperatures. DTA is a general method used to determine the cold tolerance of plants and has been used several times in pears (Kaya et al., 2020; Montano et al., 1987; Quamme, 1976; Rajashekar et al., 1982). Thus, coffee extract treatment in pears could be a relatively inexpensive way to reduce the impact of cold temperatures on pollen formation and development. Calcium required for pollen maturation accumulates in the tapetum of the anther in the form of calcium oxalate (Gebura and Winiarczyk, 2016; Hardy and Stevenson, 2000); caffeine, a major component of coffee extracts, is known to inhibit ice nucleation and freezing by binding to calcium oxalate (Kawahara et al., 2017). The presence of calcium oxalate has been reported in other anther organs, including the connective tissues and cells beneath the stomium in petunias (Petunia hybrida Vilm.) (Iwano et al., 2004) and anther endothecium in Helianthus annuus L. and H. tuberosus L. (Meric and Dane, 2004). In pears, freezing may be inhibited by the binding of caffeine in the coffee extract to calcium oxalate in the anthers. In our study, caffeine’s inhibition of low-temperature damage to pollen was evaluated using an incubator pre-cooled to −3°C. The time for the flower bud surface to change from 15°C to −3°C in such a pre-cooled incubator is about 2 h (unpublished data), which is much faster than in the field, where it takes about 15 h for the temperature to fall from its highest to its lowest point. Slower cooling rates are shown to delay the freezing of flower buds in peach (Prunus persica Batsch.) (Ashworth, 1982) and blueberry (Vaccinium corymbosum L.) (Flinn and Ashworth, 1994). The effect of coffee extracts under field conditions also needs investigation.
ConclusionThe most cold-sensitive flower bud stage in Japanese pear ‘Kosui’ pollen was the scale-separation stage or stage I, with pollen in that stage generally ranging from the pollen mother cell to the tetrad stage. Anther development abnormalities were observed in the ‘Kosui’ flower bud that was treated at −3°C for 10 h, including tapetum hypertrophy, cell debris, and locule shrink. Among seven pear cultivars, ‘Hosui’ pollen was the most tolerant of low temperature exposure at the scale-separation flower bud stage. In the laboratory environment, the application of coffee extract before cold treatment delayed the freezing of flower buds and preserved the pollen germination rate.