2023 年 92 巻 2 号 p. 162-170
2023 年 92 巻 2 号 p. 162-170
Winged bean seeds require imbibition treatment to ensure fast and uniform germination. Seed soaking has been commonly used; however, it is unclear if this is a safe method for winged bean seeds. Solid matrix priming (SMP) is an imbibition treatment that combines seeds, a matrix and water in a specific ratio. It allows seeds to imbibe under controlled water uptake. We investigated the effect of imbibition treatments on seed germination of white winged bean. Soaked seeds had significantly reduced germination and emergence as a result of imbibition injury due to rapid imbibition. SMP at a seed: vermiculite: water ratio of 7:12:9 (w/w/w) was the most effective treatment that allowed seeds to imbibe slowly without the occurrence of imbibition injury. SMP significantly reduced the mean germination time from 4.63 days to 2.01 days, and mean emergence time from 7.21 days to 5.78 days, compared to the control, as well as having a high germination rate of 98% and uniform emergence. The fast imbibition rate of white winged bean seeds was likely the result of cracks present on the permeable seed coat.
Winged bean (Psophocarpus tetragonolobus (L.) DC.) is a tropical leguminous vegetable crop grown for its edible green pods, and the tuberous roots, dry and green seeds, young leaves, and flowers are also sometimes eaten (Claydon, 1975; Eagleton, 1999). Winged bean is commonly grown in tropical countries such as Malaysia, Indonesia, Myanmar and Sri Lanka as the plant thrives in hot and humid climates (NAS, 1975). In recent years, winged bean green pods have gradually become a popular vegetable in Taiwan. To ensure uniform seed hydration and germination, imbibition pre-treatment such as seed soaking and priming is often necessary. However, the scientific research on winged bean is mostly related to the nutritional composition of the mature seeds and the plant cultivation, while imbibition treatment on seed germination has not been investigated.
Soaking is an ancient technique commonly used to enhance seed germination quality prior to sowing. However, a lack of controlled water uptake and imbibition rate sometimes result in imbibition injury. Imbibition injury happens when seeds imbibe too rapidly, especially when the seed moisture content is low. Seeds damaged by imbibition injury have a low germination rate (Thornton et al., 1990), a high percentage of abnormal seedlings (Toledo et al., 2010), and increased solute leakage (Shereena and Salim, 2006). Under wet and cold conditions, necrosis was first observed in the meristematic regions of the radicle, plumule, and some of the surrounding tissue of the seeds (Harrison, 1973). Winged bean seeds are relatively large, and large seeded legumes are particularly vulnerable to imbibition injury (Powell, 1998). For example, imbibition injury commonly occurred in seeds of soybeans (Oliveira et al., 1984), chickpeas (Legesse and Powell, 1992), cowpeas (Abdullah et al., 1992), fava beans and peas (Rowland and Gusta, 1976).
The seed imbibition rate is also closely related to the integrity of its seed coat. The seed coat acts as an important barrier to water entry for the embryo and slows down its imbibition (Powell and Matthews, 1979). Seed coat damage can occur during harvest or a storage period, thereby increasing the imbibition rate and causing seed injury during imbibition (McDonald et al., 1988). Pea (Pisum sativum L.) seeds with a fast imbibition rate resulted in abnormal (morphological defects), ungerminated, or dead seedlings, compared to seeds with a slow imbibition rate (Veselova et al., 2003). When pea seeds without a seed coat imbibed in water, cotyledonal tissue damage caused by rapid water uptake was observed, and such damage was reduced when the imbibition rate was reduced (Powell and Matthews, 1978).
During seed imbibition, the ‘pre-germinative metabolism’ is activated, such as the de-novo synthesis of nucleic acids and proteins, ATP production, accumulation of sterols and phospholipids, activation of DNA repair and antioxidant mechanisms (Paparella et al., 2015). Soaked seeds of the common bean (Phaseolus vulgaris) had a noticeably lower germination rate compared to unsoaked seeds (Orphanos and Heydecker, 1968). As a countermeasure, solid matrix priming (SMP) can be used, in which seeds are mixed with water and a non-toxic solid material with low matric potential such as vermiculite. SMP allows control of the imbibition and moisture content of seeds (Harman and Taylor, 1988), as well as allowing air circulation (Paparella et al., 2015).
Currently, there is a lack of information on safe imbibition treatments for winged bean seeds. This undoubtedly poses a barrier to achieving high quality seedlings in commercial cultivation. The objective of this study was to evaluate imbibition treatments suitable for winged bean seeds. Therefore, the following were investigated: (1) The effectiveness of imbibition methods on seed germination and emergence; (2) The effect of seed soaking on imbibition injury; and (3) The imbibition rate in relation to the seed coat structure.
A white variety winged bean ‘Chuilu’ bought from the PoYu Trading Co., Ltd was used. In the text, we refer to this winged bean variety simply as white winged bean. This particular variety of winged bean was chosen because our pre-experiment showed its seeds were very vulnerable to imbibition injury. The initial seed moisture content was 9.23% (fresh basis). The seeds were weighed and only seeds between 0.29 and 0.40 g/seed were selected and used in the experiment.
Imbibition treatments Noguchi germinatorThe germinator (7 cm × 10 cm × 1.5 cm) was soaked in water for 15 minutes, then transferred into a plastic box (8 cm × 12.5 cm × 4 cm) with a closed lid. Seeds were placed on the germinator, to which water was added up to half of the height of the germinator, and incubated at 30°C in a growth chamber. Seeds imbibed through the capillary action of water through the germinator. The water level was maintained daily at half the height of the germinator.
SMPSeeds were mixed with vermiculite and water at a seed: vermiculite: water ratio of 7:12:9 (w/w/w) and placed in sealed plastic boxes. The sealed boxes were incubated at 23°C in a growth chamber. At the end of the treatment, seeds were sieved out from the mixture.
Soaking: Seeds were placed in a beaker containing free-flowing water at a room temperature of 27°C.
In the germination and emergence tests, seeds were treated in the Noguchi germinator for two days, SMP for two days, and were soaked for eight hours, while untreated seeds were used as a control.
Imbibition curveSeeds were treated with the above imbibition treatments. Seeds in the control were placed in a 90-mm Petri dish lined with two sheets of Whatman no. 1 filter paper moistened with 4 mL of water, incubated at 30°C in a growth chamber, and water was added every day to keep the paper moist. Seeds were weighed at each of their respective imbibition periods: Noguchi germinator, 0, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.5, and 3 days; SMP, 0, 3, 6, 9, 12, and 18 hours and1, 1.5, 2, 2.5, and 3 days; Soaking, 0, 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, and 24 hours; Control, 0, 3, 6, 9, 12, and 18 hours and 1, 1.5, 2, 2.5, and 3 days. At the end of the period, the dried weight of the seeds was measured by first drying them at 103°C for 17 hours in an oven. Seeds were then weighed after being cooled down in a desiccator. Each replicate contained 15 seeds and a total of three replicates were used. The imbibition rate of seeds was calculated as the seed moisture content (fresh basis) by the following formula:
Seeds were sterilized in 3% NaOCl solution mixed with a drop of dish detergent for two minutes and washed under water for one minute. Seeds were placed in a 90-mm Petri dish lined with two sheets of Whatman no. 1 filter paper moistened with 4 mL of water, and incubated at 30°C in a growth chamber. Around 0–1 mL of water was added daily to keep the paper moist, but not wet. Germinated seeds (radicle over 1 cm) were counted daily for seven days. Seeds with abnormal, stunted root growth, cotyledon necrosis or those that were rotten were counted as abnormal. Each replicate contained 30 seeds distributed in two petri dishes and a total of four replicates were used. Germination and abnormal seeds were calculated as percentages, while the mean germination time (MGT) and uniformity (T90−T10) were calculated by the following formulas:
Seeds were sown in each cell of 72 plugs in a tray filled with a mixture of peat, perlite and vermiculite at a ratio of 8:1:1 (v/v/v). The seeds were incubated at 28/23°C with a 12 hour light period in a growth chamber. Each replicate contained 15 seeds and a total of three replicates were used. Emerging seedlings (seedling over 1.5 cm high) were counted daily for 14 days. Emergence percentage, mean emergence time and uniformity (T90−T10) were calculated as described above. At the end of day 17, seedlings from the emergence test were measured for shoot length, diameter, and fresh weight, as well as root fresh and dried weight. Each replicate contained three seedlings and a total of three replicates were used.
Abnormal seed test for soaked seedsSeeds were soaked in free-flowing water for 0, 1, 2, 4, 6, and 8 hours. After soaking, seeds were subjected the germination test described above for seven days at 30°C. Each replicate contained 45 seeds (15 seeds/petri dish) and a total of three replicates were used. Normal seeds (normal root growth, cotyledons and embryonic axis), injured seeds (necrosis in the connective tissue between cotyledons and embryo axis) and abnormal seeds (abnormal root growth, severe necrosis, detached embryo axis from cotyledons or rotten) were counted at the end of day 7 and calculated as a percentage.
Electrical conductivityOne seed was soaked in a tube filled with 15 mL of deionized water for 2, 4, 6, 8, 12, 16, 20, and 24 hours at 27°C. Each replicate contained one tube and a total of three replicates were used. The electrical conductivity (EC) of the soaking water was measured by a conductivity meter (SevenCompact S230; Mettler Toledo) and calculated by the following formula:
The hilum, whole seed or seed coat (whole seed excluding hilum) were covered in a layer of Vaseline and soaked in water. After one day of soaking, fully imbibed seeds (size increased more than half the original size) were counted, and expressed as a percentage of the total seed number. Each replicate contained 15 seeds and a total of three replicates were used.
Seed coat structural observation by scanning electron microscopy (SEM)Permeable and hardseeded white winged bean seeds were used. Permeable seeds were selected by searching for seeds with a vertical crack on the lens (more than 90% of the seeds had this crack). Hardseeds were selected by first soaking seeds in water for one day, and seeds that did not imbibe any water (no size increase or wrinkled seed coat) were selected as the desired white winged bean hardseeds. The samples were coated with gold and examined with a scanning electron microscope (S-3000N; Hitachi) at 15 kV.
Statistical analysisAll data are represented as the mean value of replications. Analyses were performed using the software SAS Enterprise Guide 7.1 and the mean variance of the data was analyzed using Fisher’s least significant difference (LSD) test at a 0.05 probability level. Germination and emergence percentages were arcsine transformed prior to analysis to improve normality.
The imbibition rates of white winged bean seeds by treatments, from the highest to lowest, were: soaking, SMP, petri dish, and the Noguchi germinator. For example, in order to reach 45% seed moisture content, the time required for soaking was seven hours, while that of SMP, petri dish and the Noguchi germinator were 1.25, 2, and 2.5 days, respectively (Fig. 1). Seeds in all the treatments had a noticeably decreased imbibition rate after reaching 40% moisture content, with the exception of the Noguchi germinator. Seeds in the Noguchi germinator had a mostly steady imbibition rate throughout the course of imbibition.

Moisture content of white winged bean seeds imbibed in a Petri dish, a Noguchi germinator at 30°C, solid matrix priming (SMP; 7:12:9) at 23°C, or soaked in water at 27°C. Error bars indicate the standard deviation of the means.
In the germination test, soaking seeds for eight hours had detrimental effects, with only 55% germination compared to 97–98% in the other treatments (Table 1). Soaking treatment also resulted in 45% abnormal seeds, while the other treatments had close to 0%. Seeds treated by all imbibition methods had a significantly lower MGT and higher uniformity (lower T90−T10) than the control. Seeds in SMP had the lowest MGT of 2.01 days among the treatments, but also had 98% germination. The MGT of the seeds in the control was 4.63 days.

Effects of imbibition treatments on the germination percentage, abnormal seed percentage, mean germination time (MGT) and uniformity (T90−T10) of white winged bean seeds germinated in petri dishes at 30°C for seven days.
In the emergence experiment, soaking significantly reduced the emergence percentage to 8% compared to 73% in the control (Table 2). The Noguchi germinator and SMP-treated seeds increased the emergence percentage to 98%. SMP treatment reduced MET to 5.78 days, compared to 7.21 in the control. Seeds treated with SMP and the Noguchi germinator also emerged more uniformly, with most seeds emerging within 3.12 days compared to 4.77 days in the control.
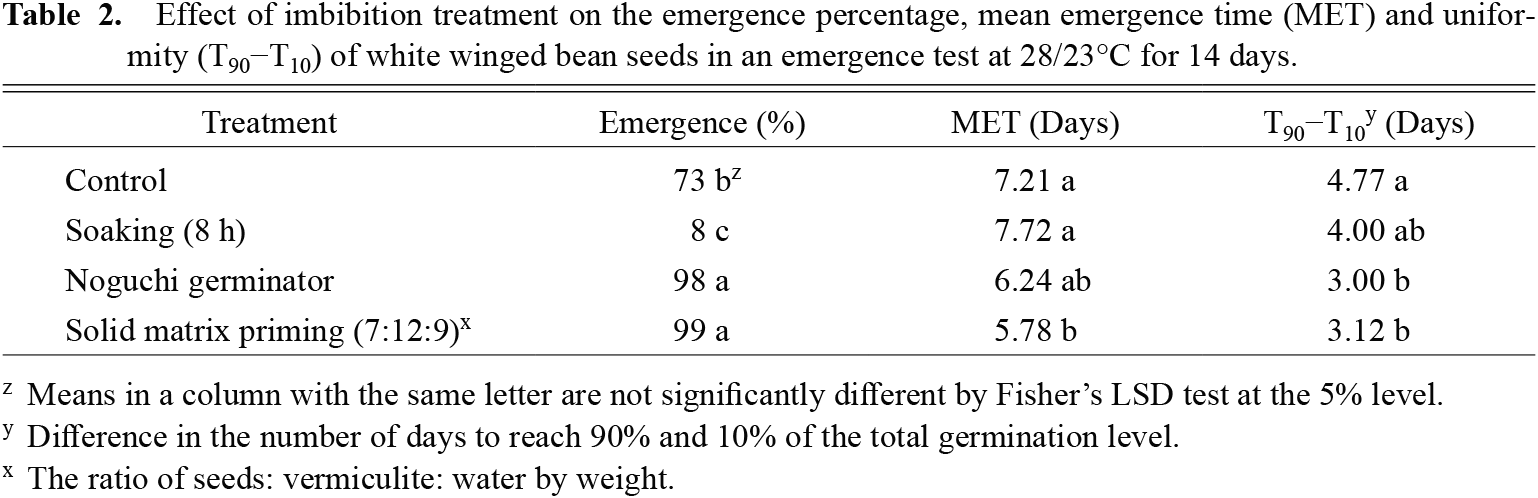
Effect of imbibition treatment on the emergence percentage, mean emergence time (MET) and uniformity (T90−T10) of white winged bean seeds in an emergence test at 28/23°C for 14 days.
Soaking significantly reduced the shoot fresh weight of seedlings to 1.85 g compared to 2.12 g in the control (Table 3). Seedlings in the Noguchi germinator treatment had a shoot length of 17.4 cm; this was significantly higher than that of the other treatments, which was 12.2 cm or less. Soaking also significantly reduced the root dried weight of seedlings to 0.21 g compared to 0.26 g in the control. The shoot width and root fresh weight were not significantly different among treatments.

Effect of imbibition treatments on shoot fresh weight, shoot length, shoot width, root fresh and dried weight of white winged bean seedlings after 17 days of an emergence test at 28/23°C.
The percentage of normal seeds in the 1-hour soaking treatment was significantly reduced to 71% compared to 96% in the control. After soaking for eight hours, normal seeds further decreased to 16% (Table 4). Soaking seeds for two hours resulted in 30% injured seeds (seeds that germinated but showed symptoms of imbibition injury) and 21% abnormal seeds, which were significantly higher than the control. A longer soaking period resulted in a higher number of injured and abnormal seeds. Electrical conductivity (EC) of soaked white winged bean seeds increased rapidly during the first eight hours, and then increased slowly from 8 to 24 hours, finally reaching a maximum at 20–24 hours (Fig. 2).

Effect of soaking period on the percentage of normal, injured and abnormal white winged bean seeds germinated at 30°C for seven days.
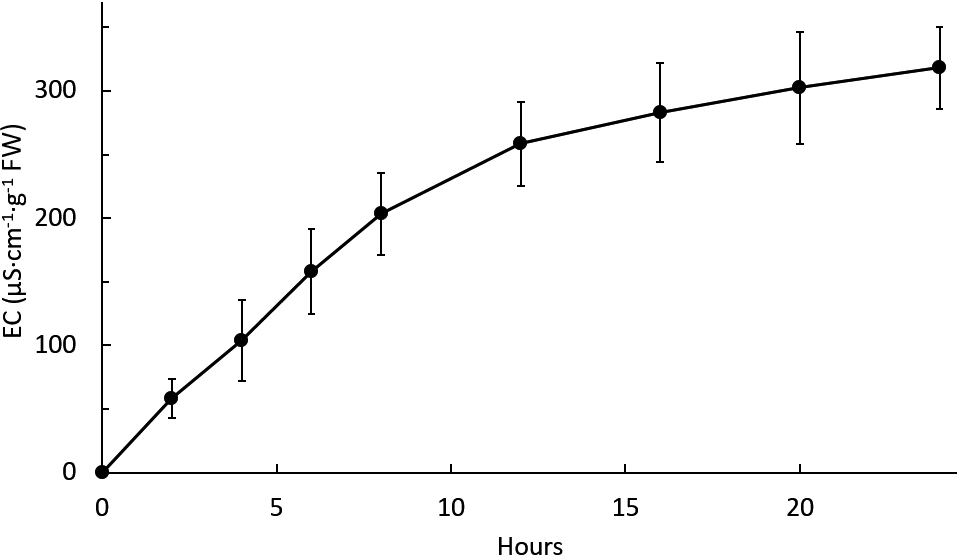
Electrical conductivity (EC) of the soaking liquid from white winged bean seeds soaked in water at 27°C. Error bars indicate the standard deviation of the mean.
Vaseline was an effective agent in the seed blocking experiment, as 0% of the seeds imbibed water when completely covered with Vaseline, compared to 98% of imbibed seeds in the control (Fig. 3). Blocking the hilum of seeds resulted in 91% imbibed seeds, whereas only 44% of seeds with blocked seed coats except for the hilum imbibed water.
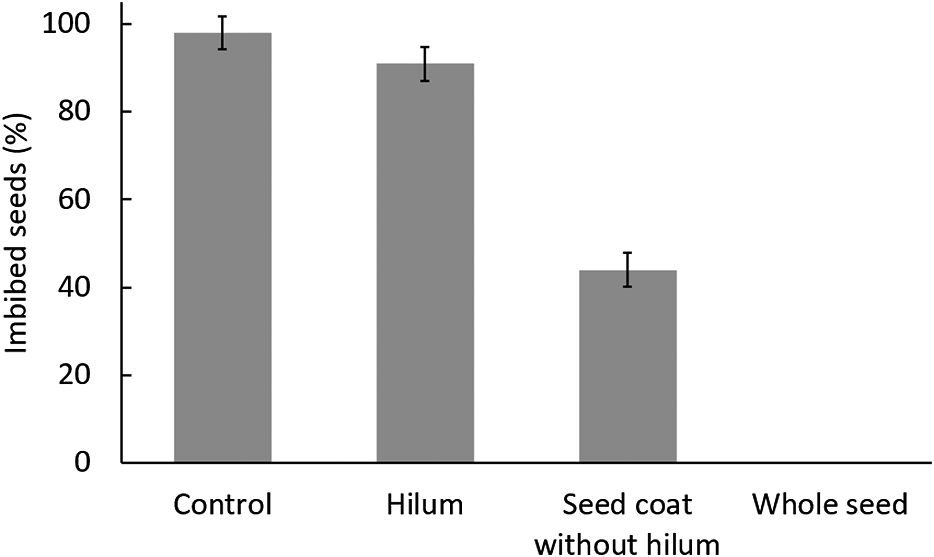
Effect of Vaseline coating of the hilum, the seed coat without the hilum or the whole seed on the percentage of imbibed white winged bean seeds soaked in water at 30°C for one day. Error bars indicate the standard deviation of the means.
The micropyle and hilum were clearly visible on the ventral side of white winged bean seeds, whereas the lens structure was only slightly visible (Fig. 4A, B). The seed micropyle was much smaller than the hilum and was very close to the hilum. The hilar fissure of permeable seeds was open (Fig. 4C) as compared to the hardseeds, where it remained closed and no visible space could be observed (Fig. 4D). Permeable seeds also had opened micropyles (Fig. 4E), whereas the micropyles remained closed and appeared as a slit in the hardseeds (Fig. 4F). In the lens region of permeable seeds, small cracks appeared near the hilum and a large vertical crack was present in the raphe region (Fig. 4G). These cracks were absent in the hardseeds (Fig. 4H). In the lens region, both permeable seeds and hardseeds had a region with a dotted-pattern on the surface (Fig. 5A, B), whereas in the hardseeds, a ring-pattern also appeared in the region very close to the hilum. As the region moved further away from the hilum, the dotted pattern disappeared and was replaced by a smooth surface. In a close-up view of the surface, the micropylar region of permeable seeds was covered by ring-patterned shapes (Fig. 5C), whereas a smooth surface was found in same region of the hardseeds and this appeared to be covered by some kind of deposit (Fig. 5D). In the lens region, permeable seeds had a very rough surface with a fabric-like pattern (Fig. 5E), while a roughly similar pattern was visible on the surface of the hardseeds; however, it appeared to be covered by a heavy layer of deposit (Fig. 5F). The surfaces of the seed coat outside the hilar region were free of visible patterns and were much smoother than inside the hilar region (Fig. 5G, H). In the hardseeds, the seed coat in this region was covered with a heavy deposit with visible small cracks (Fig. 5H), whereas in the seed coat of permeable seeds, it was free of any visible deposit or cracks (Fig. 5G). Palisade cells were visible on the small cracks of permeable seeds in the lens region and appeared to be tightly arranged (Fig. 5A, I). On the other hand, no visible palisade cells were present in the small cracks that appeared on the seed coat of the hardseeds (Fig. 5H).

Scanning electron micrographs of a white winged bean (A, C, E, G) permeable seed and (B, D, F, H) a hardseed at the (A, B) hilar region, (C, D) hilum, (E, F) micropylar and (G, H) lens. Hf, hilar fissure; Hi, hilum; Le, lens; Mi, micropyle; Vc, verticle crack. Dotted arrows indicate small cracks.
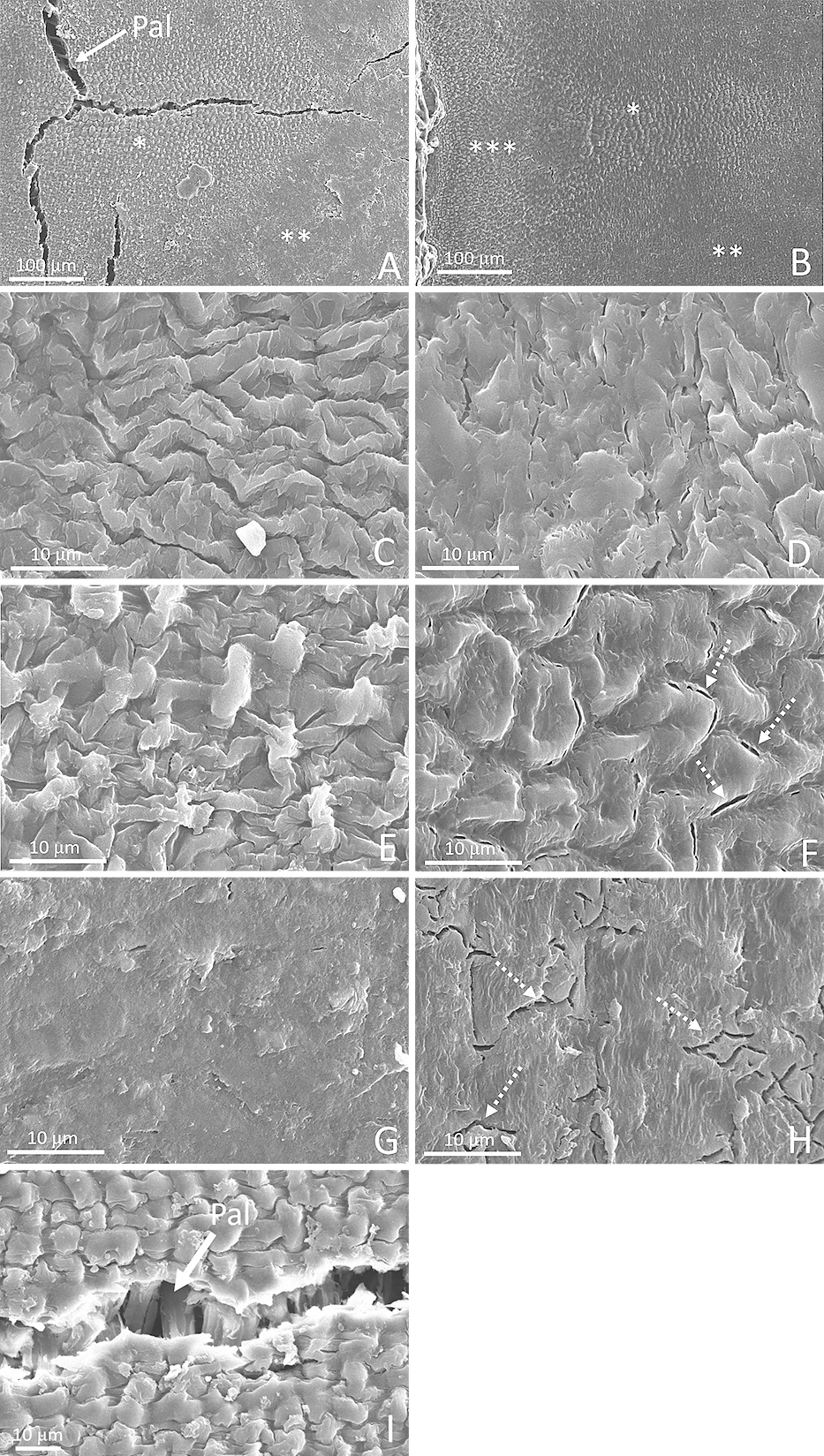
Scanning electron micrographs showing a close-up view of a white winged bean (A, C, E, G, I) permeable seed and (B, D, F, H) a hardseed at the (A, B) lens, surface of (C, D) micropylar region, (E, F) lens region, (G, H) seed coat outside hilar region, and (I) small crack. Pal, palisade cell. *, region near lens with a dot-shaped pattern; **, region near lens with a smooth surface; ***, region near hilum with a ring-shaped pattern. Dotted arrows indicate small cracks.
White winged bean seeds had very permeable seed coat, as over 90% of the seeds with a blocked hilum were able to imbibe water normally (Fig. 3). This could be because the seed coat of the permeable seeds had numerous cracks around the hilum tissue near the lens and a very large vertical crack could be observed in the lens region (Fig. 4G). Some of the cracks had penetrated into the palisade layer, as the tightly packed palisade cells were clearly visible (Fig. 5I). Peltophorum dubium and Mimosa bimucronata seeds had similar large cracks in the lens after dormancy break treatment (Geisler et al., 2016). The seed coats in this region were also rough with a detailed pattern on permeable seeds (Fig. 5E), whereas they were smooth with a less detailed pattern on the hardseeds (Fig. 5F). The seed coats near the micropyle in permeable seeds were rough (Fig. 5C), whereas the hardseeds had very smooth seed coats in the same region, seemingly covered with heavy deposits (Fig. 5D). Rough-coated cowpea seeds were observed to have a more water-permeable and thinner seed coat resulting from palisade cells with relatively thinner walls than smooth-coated seeds (Lush and Evans, 1980). Although the hardseeds had some small cracks on their seed coats further away from the hilum region, none of them appeared to penetrate the palisade layer (Fig. 5H). It was very likely that the cracks only formed on the deposit layer, as similar cracks were observed in the seed coats of soybean hardseeds (Ma et al., 2004). We speculated that the high imbibition rate in white winged bean seeds was likely the result of a permeable seed coat combined with visible cracks (Fig. 4G). A large proportion of pea seeds with high water uptake were found to have at least one crack in the seed coat (Powell and Matthews, 1979).
Besides the seed coat, the hilum and its surrounding tissue were also moderately permeable, as over 40% of the seeds with blocked seed coats (without blocking the hilum) were still able to imbibe water (Fig. 3). One possible explanation for this is that the hilum of the permeable seeds had a wider hilar fissure, that appeared to have opened (Fig. 4C), than their hardseeded counterparts (Fig. 4D). Due to the small micropyles and their proximity to the hilum, it is possible that the micropyles in some seeds were not completely covered during the Vaseline application (Fig. 4A, C). As permeable seeds also had opened micropyles (Fig. 4E) compared to the closed ones in hardseeds (Fig. 4F), this could also explain why some seeds imbibed normally even with blocked seed coats. A similar observation was made from a hardseed soybean line, which had a closed micropyle and lens unlike a softseed line (Ma et al., 2004).
Although the scale of the germination test of this study was smaller than the standard, we considered this germination test to be appropriate due to the extensive pre-experiment conducted prior to this study. The imbibition methods highlighted here were derived from our pre-experiments and only the most effective ones (the SMP and Noguchi germinator) were chosen. We did this in search of possible imbibition treatments for winged bean seeds as we could not find any reference on this subject. For example, we tried 0.5, 1, 1.5, 2, 2.5, 3, and 4-day durations for the Noguchi germinator and found that they all improved the MGT of the seeds with increasing duration, as compared to nontreated seeds, but imbibition durations of more than two days did not differ much. Thus, the two-day duration treatment was chosen for this study as it was easiest to perform (no germination occurred during treatment, no mold, and around 40% MC) while also being fast to germinate. We also tried numerous ratio-combinations for SMP before deciding to choose 7:12:9 as the treatment in this study as these seeds germinated the fastest. Since the seeds are large, we found that the vermiculite needs to be at least 12 parts (seed 7 parts) to be fully in contact with the seeds in order to hydrate them properly. Initially, we tried to re-dry the seeds after SMP, but they were difficult to germinate after being re-dried for unknown reasons. The results we obtained from our pre-experiment were similar to this study. In our pre-experiment, the Noguchi germinator and SMP-treated seeds took around two days to germinate, while nontreated seeds took more than four days. We also selected seeds that were more uniform in size (between 0.29 and 0.40 g per seed), as seeds had high variability in terms of size; roughly 1/2 of the seeds were in this particular range, while more than 1/4 were between 0.15 to 0.28 g, and less than 1/4 were between 0.4 to 0.6.
Seeds soaked in water imbibed the fastest among all imbibition treatments, reaching 50% moisture content after 12 hours (Fig. 1), which significantly reduced the mean germination and emergence times (Tables 1 and 2). However, this came at the cost of reduced fitness in seed germination and seedling parameters (Table 3). Seed soaking for white winged bean seeds was generally harmful to the seed germination and had a very low emergence percentage. There was also a significant amount of damage and reduced fitness in terms of seed germination and seedling parameters (Table 3). Soaking abnormal seeds for two hours and further soaking were only more detrimental to the seeds (Table 4). Orphanos and Heydecker (1968) found that soaking injury in seeds of common beans (Phaseolus vulgaris L.) occurred as a result of a deficient oxygen supply to the interior of the soaked seed at a critical early stage of germination. Thus, regardless of duration, seed soaking was not recommended for white winged bean seeds. In addition, seed soaking found in some seed pretreatment instructions for winged beans needs to be thoroughly re-evaluated for any possible negative effects on some winged bean varieties. We think that seeds soaked in water had a very low emergence percentage (8%) due to imbibition injury and there was a high amount of moisture in the medium, similar to waterlogging in a field. Orphanos and Heydeckter (1968) showed that imbibition injury in soaked seeds, if excess moisture was allowed to drain, could be remedied. Thus, it is also necessary to control drainage to avoid waterlogging conditions in directly-sown fields.
Seeds in the Noguchi germinator imbibed the slowest among all imbibition treatments, as the imbibition was done through water vapor (Fig. 1). The seed moisture increased in a somewhat linear and steady manner compared to other treatments, in which seed the moisture content increased rapidly upon imbibition until it reached 40%, and then slowed down during the remaining imbibition. This form of imbibition successfully reduced germination and emergence times without the occurrence of imbibition injury (Tables 1 and 2). Thus, imbibition through water vapor such as with the Noguchi germinator can be an effective and safe imbibition treatment for white winged bean seeds. However, it took significantly longer to obtain the desired seed moisture content than with the other imbibition treatments. It would also be challenging to treat seeds in bulk this way as stacked seeds may have different imbibition rates and thus require a large space.
SMP at a seed: vermiculite: water ratio of 7:12:9 was the most effective imbibition method, with the lowest MGT and a high germination percentage of 98% (Table 1). The imbibition rate of seeds in SMP (7:12:9) was very high during the first day of imbibition and was second only to soaking, reaching 43% moisture content by the end of day 1 (Fig. 1). During imbibition, as water was absorbed away from the matrix by seeds, the water potential in the matrix decreased and the seed moisture content increase was noticeably slower. Due to the limited amount of water in the matrix being available to the seeds, the seed moisture content could not surpass a fixed threshold and could be easily controlled in this way.
As seeds could be vulnerable to pathogens due to the duration and humid environment of the imbibition treatment, water with 0.05% of NaOCl could be used to reduce such incidence, while not affecting seed germination. Caution is required, however, as a higher concentration of NaOCl such as 0.1% or above had a negative effect on seed germination (data not shown). During a pre-experiment, if the primed seeds were dried back to their original moisture content, they were found to have a lower germination rate and required a longer germination time than the untreated seeds (data not shown). The loss of positive effects of primed seeds during drying has been reported (Heydecker and Gibbins, 1978) and could be the result of the loss of desiccation tolerance by imbibed seeds (Sliwinska and Jendrzejczak, 2002). SMP was proven to be a safe imbibition method as no seedlings showed symptoms of imbibition injury (Tables 1 and 2). It is a very applicable method as it only required inexpensive vermiculite as a matrix and accurate weight measurements of the components. After the treatment, the seeds were easily separated with a sieve and the vermiculite could be discarded or reused. Compared to the Noguchi germinator, SMP could be done easily in bulk, as the mixture of seed, matrix and water could be placed in a sealed plastic bag, and a similar improvement to seed germination was reported (Pandita et al., 2010). Thus, this reinforced the idea that SMP could be used as an effective imbibition method for other winged bean varieties in the future to replace seed soaking treatment. As white winged bean seeds are very vulnerable to imbibition injury, we think that imbibition treatments that do not cause injury to this variety are applicable to other winged bean varieties. Indeed, we tried the same imbibition treatments in this study on other winged bean varieties and obtained similar results, with SMP being the most effective treatment for fast germination and high rates (data not shown). We also found that seeds of other varieties in the soaking treatment were injured by a similar imbibition injury (abnormal root growth and necrosis on the embryo axis) and had cracked cotyledons, but to a lesser degree than the white variety (data not shown). From our observation, we speculate that this is due to seeds of other varieties generally having less permeable seed coats compared to the white variety.
In conclusion, soaking white winged bean seeds in water caused imbibition injury, as a result of rapid imbibition due to their permeable seed coats. The small cracks on the seed coats of the seeds observable on scanning electron micrographs were assumed to be the cause of the seed coats’ high permeability. A Noguchi germinator and SMP (7:12:9) prevented the occurrence of imbibition injury by lowering the imbibition rate of the seeds, significantly decreasing the seed germination and emergence time, while maintaining a high seed germination rate.
We would like to express our deep appreciation to Chih-Yi Chang from the Department of Forestry, National Chung Hsing University, Taiwan, for his skillful assistance on operation of the scanning electron microscope. We thank Fu-Yu Yang for her help in assisting the emergence test. We also thank Dr. Graham Eagleton for his invaluable insight and observations on winged beans.