2023 年 92 巻 3 号 p. 354-365
2023 年 92 巻 3 号 p. 354-365
Tulip (Tulipa gesneriana L.) cultivars exhibit diverse floral scents, the emissions of which are generally influenced by temperature. To fully benefit from these scents and add value to cut tulips, it is necessary to evaluate the scent emission response to temperature. This study investigated the daily emission changes of 82 volatiles, including the main scent compounds, at different temperatures (13, 18, and 23°C), in the cut tulips from eight tulip cultivars with different scents. At 23 and 18°C, the total scent emissions of each cultivar increased with flower opening and subsequently decreased with visible senescence. Floral senescence progressed more rapidly at 23°C, and the decrease in total scent emission occurred most rapidly at 23°C. Scent emissions at 18°C tended to be lower than at 23°C. The vase life of the cut tulips was most prolonged at 13°C owing to the slow senescence; however, scent emissions remained low after the flower opening. These results indicate that improving both tulip scent emission and vase life under constant temperature conditions is difficult. It was observed that scent composition changed before and after flower opening, and with floral senescence. In contrast, temperature had little effect on scent composition. Cut tulips stored at a low temperature (10 or 15°C) over four and a half days and then transferred to room temperature (20°C) had a longer vase life than tulips maintained at room temperature, due to delayed senescence. In addition, scent emissions were low at low temperatures, but increased rapidly upon transfer to room temperature. Therefore, storage of cut tulips at low temperatures may lead to increased scent emissions upon transfer to room temperature. These findings imply that low-temperature storage of cut tulips before sale contributes to both vase life and a rich scent after sale. The results reported here demonstrate the improvement of cut tulip scent emissions by appropriate temperature management.
Tulip (Tulipa gesneriana L.) cultivars of various colors and shapes are widely used in Japan for cut-flower production. The exact annual production of tulip cut flowers is unknown as the Ministry of Agriculture, Forestry and Fisheries of Japan does not include it in the statistics. However, it is estimated that there are approximately 75 million cut flowers harvested from winter to spring and shipped by dry transport (Ichimura, 2013).
In a questionnaire survey on flower scents conducted among 870 general consumers in Ibaraki, Japan, approximately 30% of respondents stated that they would like to add scent to tulips (Kishimoto, 2012). This percentage was higher than the response received for chrysanthemums and lilies (approximately 14 and 13%, respectively) and was among the highest, alongside carnations and roses. This indicates that the demand for tulip scents in Japan is relatively high. In contrast, the same survey revealed that over 90% of the respondents stated that they did not know the scent of tulip flowers. However, tulips show a variety of scents. Oyama-Okubo and Tsuji (2019) investigated and classified the scents emitted by the cut flowers of 137 tulip cultivars into nine groups, based on primary scent components. For example, the tulip cultivars ‘Ballerina’ and ‘Sanne’ have sesquiterpene β-ionone- and monoterpene linalool-based scents, respectively, which are grouped into the fruity category. Sensory evaluation tests using cut flowers from these two cultivars show that 70% of the monitors rate the scents as preferred when there are sufficient scent emissions (Kishimoto et al., 2018). Therefore, ensuring sufficient tulip scent emissions has great value-adding potential for the cut flowers.
The quantity and composition of floral scent emissions change during anthesis; furthermore, emission quantity usually decreases with flower senescence (Negre et al., 2003; Underwood et al., 2005), although it may also decrease before senescence, as observed in cut flowers of carnations (Dianthus caryophyllus L.) (Kishimoto and Shibuya, 2021). The young and mature flowers of both jasmine (Jasminum grandiflorum L.) and raspberry (Rubus idaeus L.) have different scents (Pragadheesh et al., 2017; Robertson et al., 1995) owing to changes in terpenoid content, the main scent components of the flowers.
Floral scent emission is also affected by temperature. In the wild petunia, Petunia axillaris (Lam.) Britt., scent emission increased within the range of 20 to 30°C (Sagae et al., 2008). Similarly, cut carnation scent emission was higher at 28°C than that at 10 and 15°C, but the reduction of scent emission also began earlier at 28°C (Kishimoto, 2022). The vase life of cut flowers decreases with temperature increase, as is noticeable in tulips (Cevallos and Reid, 2001; Ichimura et al., 2011). Therefore, temperature variation induced changes in tulip vase life may also affect scent emission. Changes in scent emission quantity and composition are caused by various factors, including senescence and temperature, and affect the intensity and quality of floral scents. To use floral scent as an added value in cut flowers, it is necessary to elucidate the effects of flower senescence and temperature on scent emission. However, daily changes in the quantity and composition of scent emissions during anthesis are only known for the cultivar ‘Sanne’ (Kishimoto and Watanabe, 2023), and the effects of temperature on scent emission are unreported.
The diverse scents of tulips are primarily composed of aromatic benzenoid compounds, fatty acid derivatives, and terpenoids (Oyama-Okubo and Tsuji, 2019), and may vary in response to temperature. In this study, eight cultivars, with benzenoids, fatty acid derivatives, monoterpenes, or sesquiterpene as the main scent components, were selected to investigate the effect of temperature on the scent emission of cut tulip flowers.
It is difficult to accurately identify and quantify all scent compounds in tulip flowers, of which at least 183 scent components have been reported (Oyama-Okubo and Tsuji, 2019). Oyama-Okubo and Tsuji (2019) grouped tulip scents according to composition, listing eight benzenoids, seven fatty acid derivatives, and eleven terpenoids as the most important scent-related components of tulips. Along with the above-mentioned scent compounds, 82 volatiles were investigated (Table S1), including the main scent compounds of each cultivar, to obtain an overview of changes in cut tulip scent. As these 82 compounds account for over 80% of the total scent emissions, they can be reliably used to evaluate changes in tulip scents under different environmental conditions. Based on the investigation of these 82 scent compounds, this study examined the changes in scent emission quantity and composition of scent emission during anthesis in cut tulips of eight cultivars at 23°C, a common quality confirmation test temperature used for cutting flowers (Ichimura et al. 2011; Pun et al. 2016; Shimizu-Yumoto et al. 2020). Furthermore, we performed the same assessment at 13 and 18°C to compare the effects of different temperatures on scent emission. Finally, using cut tulips from cultivars ‘Ballerina’, ‘Kung-Fu’, and ‘Sanne’, which differ by main scent components, we investigated how scent emission is affected by temperature changes. This test was conducted at 10, 15, and 20°C, as 10 and 15°C correspond to the common display refrigerator temperature for cut flowers, whereas 20°C is the winter indoor temperature recommended by the Japanese government (Agency for Natural Resource and Energy, https://www.enecho.meti.go.jp/category/saving_and_new/saving/general/howto/airconditioning/index.html). Our study revealed the relationship between scent emission and temperature in cut tulips. In addition, we discuss the possibility of promoting the scent of cut tulips through temperature management after harvest.
The cut tulips of eight cultivars (Table 1) used in the constant temperature test were purchased from several commercial growers in Niigata or Toyama, Japan. These tulip flowers were not treated with any preservatives and were packed in cardboard boxes (H 27 × W 27 × L 79 cm or similar) containing 30–40 flowers each, then shipped by dry transport in January and February from 2015 to 2017. Neither the temperature nor relative humidity were investigated during transport.
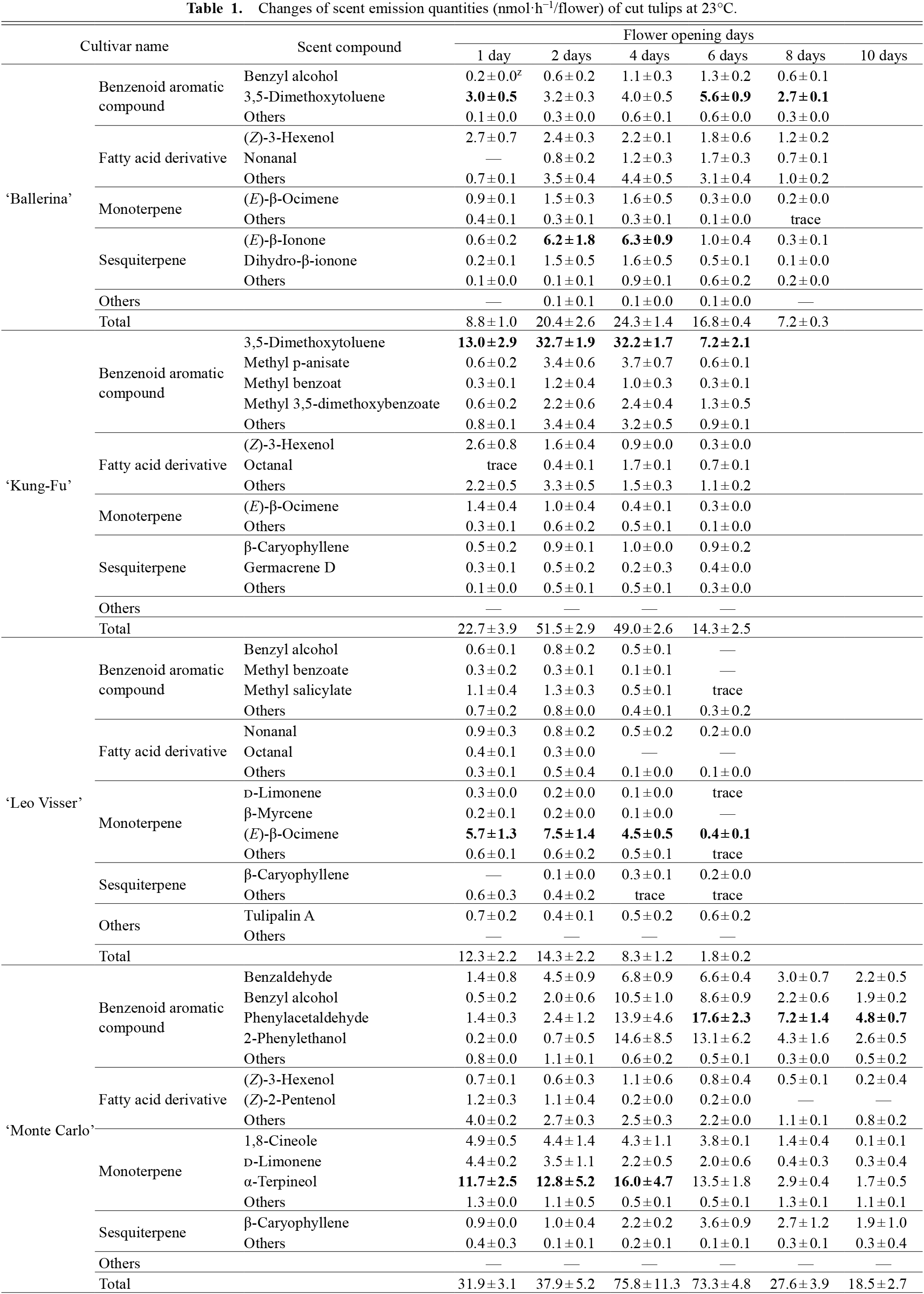
Changes of scent emission quantities (nmol·h−1/flower) of cut tulips at 23°C.
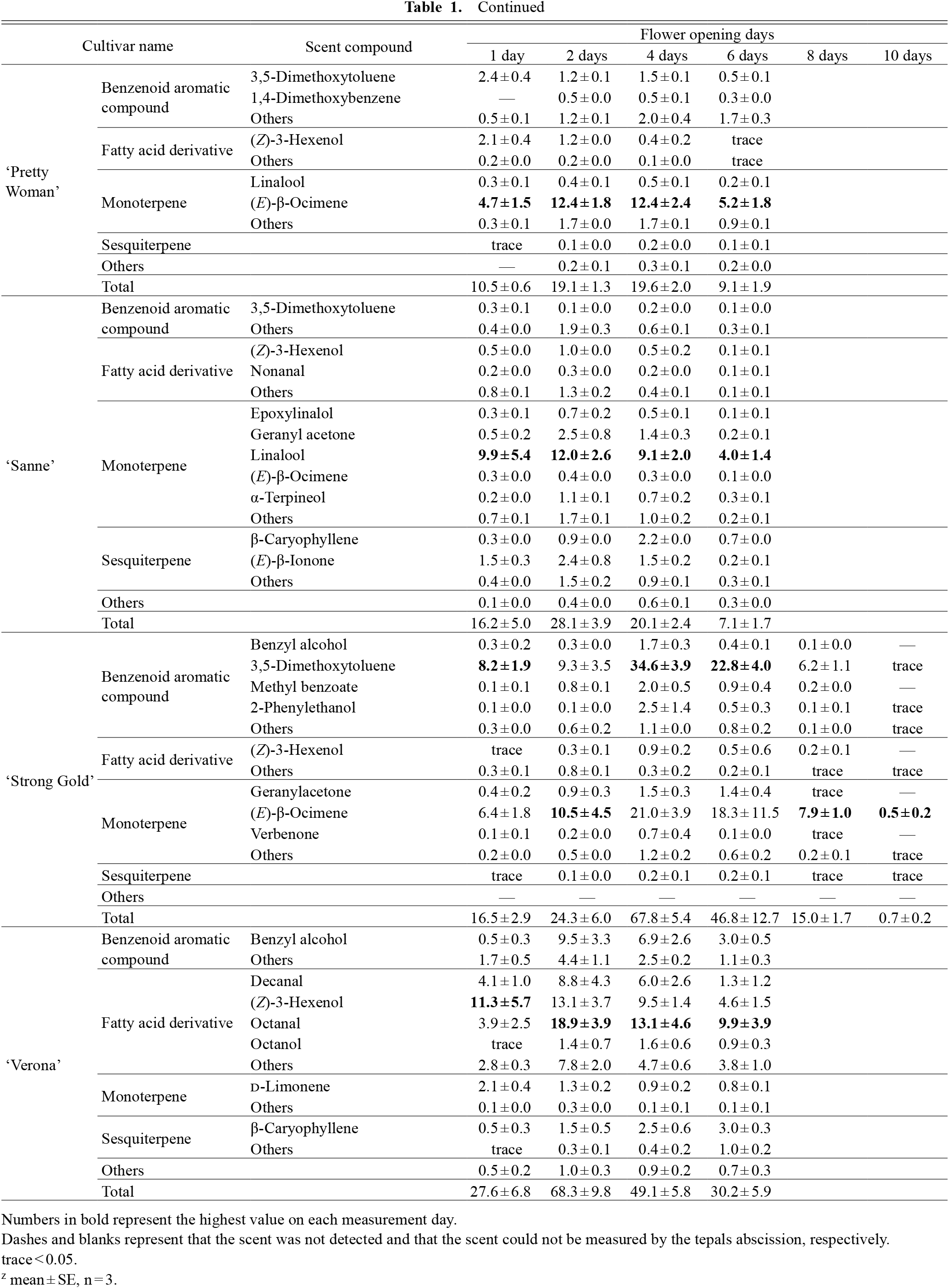
Continued
Tulip flowers of cultivars ‘Ballerina’, ‘Kung-Fu’, and ‘Sanne’, used for the temperature change test, were cultivated at the Horticultural Research Center, Niigata Agricultural Research Institute (37°59' N, 139°17' E) in Kitakambara, Japan. The bulbs, rooted by vernalization (Kishimoto and Watanabe, 2023), were planted in containers (H 25 × W 40 × L 60 cm) filled with potting soil mixed with coconut husk fiber and peat moss for cultivation in a glass greenhouse in which day and night temperatures were maintained at 18 and 13°C, respectively. No fertilizers were used. Flowers were harvested when the buds were 50–70% colored, ‘Ballerina’ and ‘Sanne’ were harvested in January and February 2017, respectively, and those of ‘Kung-Fu’ were harvested in January 2018. Thirty cut flowers, approximately 40 cm long, were placed in a glass flask containing tap water and stored at 5°C in the dark. After 3 h, all 30 cut flowers were wrapped in dry newspaper, packed in a cardboard box (H 21 × W 49 × L 81 cm), and maintained at 5°C and 30% relative humidity for approximately 1 day. The flowers were sent by a private courier to the Institute of Vegetable and Floriculture Science, NARO (36°02' N, 140°05' E), in Tsukuba, Japan. Using a data logger, temperature and relative humidity during transport were measured at 2–9°C and > 80%, respectively with a transit time of approximately 19 h.
Temperature treatment 1. Constant temperature testFlower incubation conditions were in accordance with Ichimura et al. (2011). Briefly, each cut flower was cut back to a stem length of approximately 30 cm and placed in a glass flask containing 500 mL of distilled water. Subsequently, they were kept under a 12 h light-dark photoperiod at a light intensity of approximately 10 μmol·m−2·s−1 and relative humidity of 70%. To ensure similar cut flower physiological conditions, they were stored at 23°C for 3 h (Kishimoto, 2022). Then divided into three groups and maintained at 13, 18, and 23°C, respectively.
2. Temperature change testCut tulips were stored under the same conditions as the constant temperature test, except for temperature. To ensure similar cut flower physiological conditions, they were stored at 10°C for 3 h. Subsequently, they were divided into three groups and kept at 10, 15, and 20°C, respectively. After four and a half days, sufficient time for detectable maturity differences in cut tulips at 20°C and other temperatures, the temperature of all groups was set to 20°C.
Collection of emitted scents and analysis by gas chromatography-mass spectrometry (GC-MS) analysisThe emitted tulip flower scents were collected by the dynamic headspace method (Kishimoto and Watanabe, 2023). Collections were consistently performed at 09:30 h to reduce circadian rhythm effects on scent emission (Yang et al., 2020). Tulip flowers were carefully wrapped in 1-L Tedlar bags (GL Science Inc., Tokyo, Japan) and sealed with tape. A constant stream of air (500 mL·min−1) was filtered through activated charcoal and piped into the bags. Volatiles were collected over 1 h using a Tenax-TA tube (180 mg, 60 × 80 mesh; Gerstel GmbH & Co. KG, Mülheim, Germany) and analyzed by gas chromatography-mass spectrometry (GC-MS) using an Agilent 6890 N GC system with an Agilent 5930 N mass selective detector (Agilent Technologies, Santa Clara, CA, USA). The temperature increase setting for Thermal Desorption System 2 (Gerstel GmbH & Co., KG) was 60°C·min−1, with an initial temperature of 30°C, and final temperature of 250°C, which was maintained for 10 min. The GC instrument was equipped with a cooled injection system (CIS; Gerstel GmbH & Co.) and a DB-WAX capillary column (30 m length, 0.25 mm inner diameter, and 0.25 μm film thickness; Agilent Technologies). The CIS was set to splitless mode, cryofocusing set at −50°C, the temperature increase rate was 12°C·s−1, and a final temperature of 300°C. Helium was the carrier gas, at a flow rate of 1 mL·min−1. The column oven temperature program of the column oven was set to 40°C for 2 min, increased to 250°C at a rate of 5°C·min−1, and maintained at 250°C for 5 min. The injection, interface, and ion-source temperatures were 250°C. The quadrupole temperature was set to 150°C. The mass scan range was m/z 30–300, and the electron potential was set to EI 70 eV.
Candidate names for the detected compounds are listed according to the Wiley 9th/NIST 2011 library search system (Agilent Technologies). Of these, 82 compounds were identified by comparison of mass spectrums and retention times of the standards (purity > 90%, Table S1) analyzed under the same conditions. The quantity of each scent compound was calculated based on calibration curves derived from the peak areas of 5, 25, 50, 250, and 1,000 ng of each standard in the ion chromatograms. Data shown are the mean values of three independent plants for each temperature treatment.
Statistical analysisStatistical analysis of scent emissions was performed using Tukey’s test (P < 0.05) in MEPHAS (available at: https://alain003.phs.osaka-u.ac.jp/mephas/index.html) provided by the Research Institute for Microbial Diseases, Osaka University, Osaka, Japan.
Changes in the emissions of 82 scent compounds and flower appearance of cut tulips of eight cultivars at 23°C are shown in Table 1 (or Fig. 2A) and Figure 1, respectively. These 82 compounds, which contain the major scent components in each flower, accounted for 80–99% of the total peak area in the corresponding GC-MS chromatograms (Fig. S1). Most were benzenoid aromatic compounds, fatty acid derivatives, monoterpenes, or sesquiterpenes. However, the scent compositions and major scent compounds varied across cultivars.

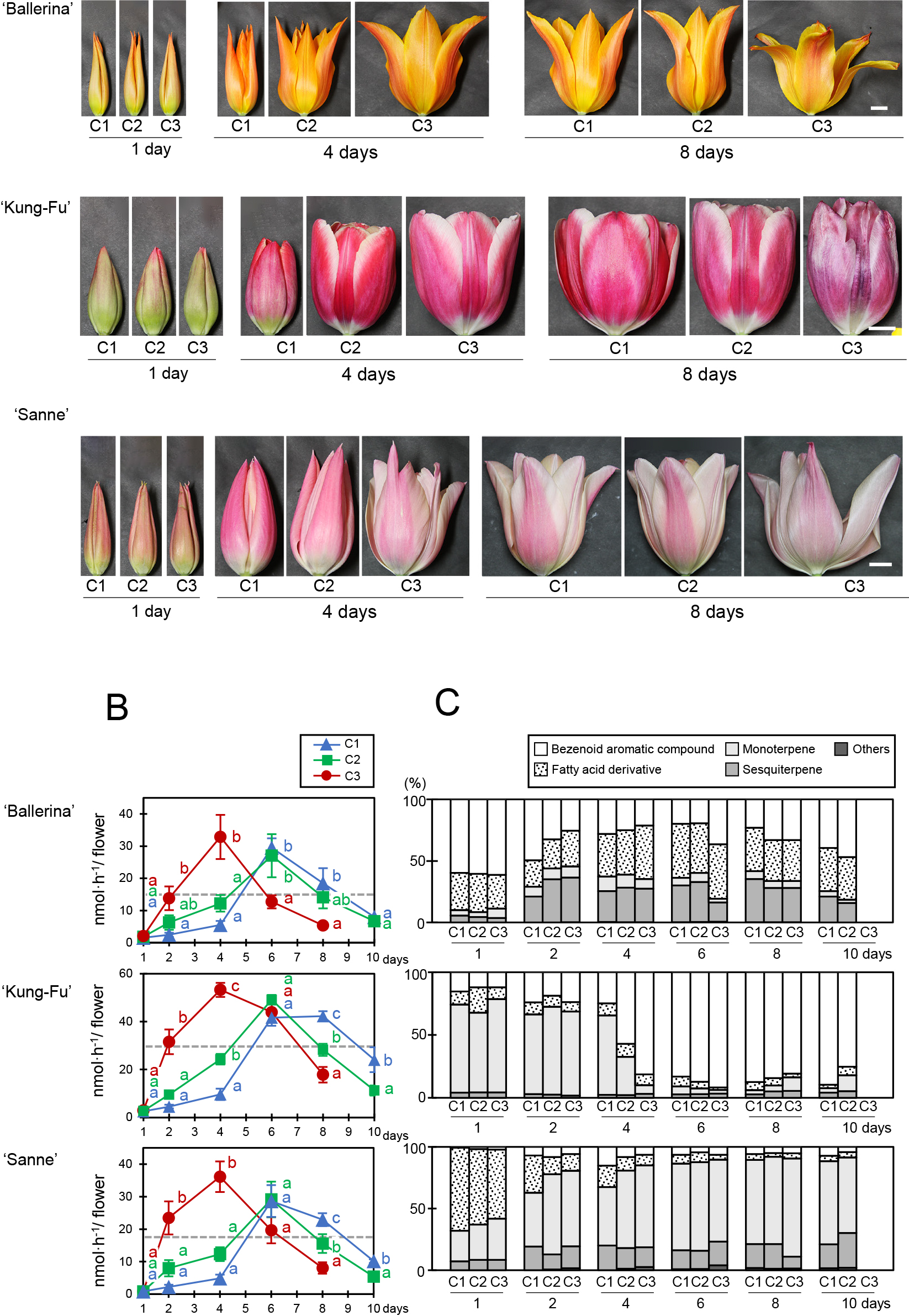
Changes in the appearance of cut tulips at different temperatures. Typical flower appearances during the test are shown with six independent cut tulips. The scale bars represent 1.5 cm.

Changes in quantities (panel A) and compositions (panel B) of scent emissions in cut tulips at different temperatures. The total emissions of the scent components shown in Table S1 were investigated. The blanks in the graphed data indicate that the scent emissions could not be measured by tepal abscission. In panel A, different lowercase letters on the same measurement day indicate significant differences in scent emissions (Tukey’s test, P < 0.05, n = 3).
No changes were detected in the types of major scent components in tulips of the cultivars ‘Kung-Fu’, ‘Sanne’, and ‘Pretty Woman’ during the observation period (Table 1). Conversely, in other cultivars, the predominant scent compounds during anthesis changed; however, certain compounds were always present in a high percentage. The emissions of the top three compounds in each cultivar always accounted for 52–95% of the total emissions.
Regarding the scent emission composition, composition ratios in several cultivars changed daily owing to increases or decreases in the major compounds. Thus, for example, in ‘Ballerina’, the proportions of benzenoids and fatty acid derivatives were high on the 1st day. Sesquiterpenes showed a high proportion on the 2nd and 4th days (Fig. 2B). After the 6th day, the proportion of sesquiterpenes decreased again. These changes in ‘Ballerina’ were primarily caused by an increase or decrease in β-ionone, one of the main scent compounds (Table 1). Particularly for ‘Monte Carlo’, the proportion of monoterpenes was high on the 1st and 2nd day, while the benzenoid proportion was high after the 4th day (Fig. 2B). This change was also primarily due to an increase in limited benzenoids, including phenylacetaldehyde (Table 1). Remarkable changes in scent compositions were also observed for ‘Strong Gold’ on the 10th day and ‘Leo Visser’ on the 6th day (Fig. 2B). These scent composition changes appear to be synchronized with flower opening or visible (i.e., discoloration or wilting of the tepals) flower senescence (Fig. 1; Table S2).
In all the cultivars, total scent emissions increased rapidly once, followed by a decrease (Fig. 2A). Rapid increases were observed during the opening of the tepals (Fig. 1). Conversely, emissions decreased with flower senescence. For cultivars with early tepal abscission, such as ‘Kung-Fu’, ‘Leo Visser’, or ‘Sanne’ (Table S2), scent emission decreases also started early. Thus, flower senescence development was different for each cultivar (Fig. 1; Table S2), as was the subsequent onset of the decrease in scent emission (Fig. 2).
Comparison of scent emissions under different temperaturesComparisons of changes in flower appearance, total scent emission, and scent composition in cut flowers from eight cultivars at 13, 18, and 23°C are shown in Figure 1, Figure 2A, and Figure 2B, respectively. Before this comparison, the scent emissions of the cut flowers were investigated at 23°C, with no significant difference observed among cultivars (day 1 in Fig. 2). Visible flower senescence with discoloration, wilting, or tepal abscission progressed faster at higher temperatures (Fig. 1; Table S2).
The pattern of change in total scent emissions was temperature dependent (Fig. 2A). In all the cultivars, the total emissions at 23°C increased most rapidly and decreased earliest. Furthermore, the highest emissions were detected at 23°C. Exceptionally, in ‘Sanne’ and ‘Verona’, the highest emissions recorded at 18°C (day 4 in Fig. 2A) equaled those recorded at 23°C (day 2). Total scent emissions at 18°C, excluding those of the ‘Leo Visser’, gradually increased and then decreased slowly compared to those at 23°C. Therefore, the total emissions for storage at 18°C significantly exceeded those at 23°C. The number of days where total emissions at 18°C exceeded those at 23°C differed among cultivars. At 13°C, differences in total scent emissions were relatively minor and remained low after the flower opening.
Most scent compositions were not remarkably affected by differences in temperature (Fig. 2B). In contrast, the scent composition of ‘Leo Visser’ on the 6th day and ‘Strong Gold’ on the 10th day at 23°C differed from those at 13 and 18°C. However, the total scent emissions at those times were low (Fig. 2A).
Effect of temperature change on scent emissionThe effect of constant temperature and that of temperature changes on scent emissions was investigated in the cut tulips of cultivars ‘Ballerina’, ‘Kung-Fu’, and ‘Sanne’ (Fig. 3). After dry transport, the scent emissions on the second day after harvest were investigated at 10°C. We found no significant differences in scent emissions (day 1 in Fig. 3); then tulips were kept at 10 or 15°C for four and a half days before being transferred to 20°C [conditions 1 (C1) and 2 (C2), respectively]. Furthermore, tulips were kept at 20°C as controls [condition 3, (C3)]. Flower senescence, manifested by discoloration or wilting of the tepals, progressed earlier in C3, followed by C2 and C1 (Fig. 3A; Table S3).
Changes in flower appearance (panel A), amount of scent emissions (panel B), and composition of scent emissions (panel C) in cut tulips under different temperatures. First, the cut flowers were kept at 10°C for 3 h, and their scent emissions were investigated (day 1). Subsequently, flowers were grouped into three conditions and kept at 10°C (C1 = Condition 1), 15°C (C2 = Condition 2), or 20°C (C3 = Condition 3), respectively. After four and a half days, the temperature was set at 20°C for all flower groups. The total emissions of the scent components shown in Table S1 were investigated. In panel A, typical flower appearances during the test are shown using six independent cut tulips. The scale bars represent 1.5 cm. In panel B, different lowercase letters on the same measurement day indicate significant differences in scent emissions (Tukey’s test, P < 0.05, n = 3). In our previous study, the scent intensities of cut tulips of these three cultivars were categorized by 394 monitors as “very scented”, “scented”, “slightly scented”, or “unscented”, (Kishimoto et al., 2018). The dotted lines in panel B indicate the lower limit at which over 70% of the monitors positively evaluated “very scented” or “scented”. In panels B and C, the blanks in the graphed data indicate that the scent emissions could not be measured by tepal abscission.
When comparing the total scent emissions for each experimental condition, emissions in C3 increased earlier than those in C1 or C2; furthermore, they decreased earlier in C3 than in C1 or C2 (Fig. 3B). The scent emissions in C1 and C2 remained low and steady at 10 and 15°C, but increased rapidly from the 6th day onward, half a day after the temperature change to 20°C. In ‘Ballerina’, there was no differences in the change of scent emissions between C1 and C2; however, in ‘Kung-Fu’ and ‘Sanne’, scent emissions were higher in C1 than those in C2 from the 8th day onward.
The three cultivars showed different scent compositions before and after flower opening or coloring (Fig. 3A, C). In ‘Ballerina’, ‘Kung-Fu’, and ‘Sanne’, benzenoids, monoterpenes, and fatty acid derivatives showed the highest proportions before flower opening, respectively. As the progress of flower opening and coloring was slow in C1, scent composition before flower opening tended to be stable, especially in ‘Kung-Fu’. Scent compositions similar to the scent emissions shown before flower opening or coloring in this test were not observed in the constant temperature test using the same tulip cultivars (Fig. 2). However, the scent compositions of the high scent emissions, after flower opening or coloring, were similar to those in the constant temperature test.
In this study, eight cultivars with different scent compositions were examined (Fig. 2B) to determine general responses of scent emission to temperature. To ensure data accuracy, we further narrowed down the target scent-related compounds to 82. The total quantity of scent-related compounds always accounted for over 80% of the GC-MS chromatograms (Fig. S1). Furthermore, the temperature conditions tested were in the range commonly used for the management/maintenance of cut flowers (Cevallos and Reid, 2001; Ichimura et al., 2011), and were sufficient to induce clear differences in cut tulip appearance and vase life. Therefore, we believe that it is valid to discuss changes in tulip scent based on the results of studies conducted under relatively limited conditions.
Our data demonstrated the temperature sensitivity of tulip scent emission, which agrees with the reported temperature dependency of tulip opening (Azad et al., 2004). Scent emissions consistently increased at 18°C and 23°C upon flower opening (Figs. 1 and 2A). However, at 13°C, scent emissions remained low even after flower opening. Therefore, both flower opening and scent emission are promoted by higher temperatures. However, scent emission is not consistently synchronized with flower opening.
One reason for low scent emission at low temperatures is that the physical vaporization of scent compounds from cuticles on the tepals surface may not be especially active at low temperatures. In petunia flowers, scent emission increased with increasing temperature (Sagae et al., 2008), with approximately half of the floral scent components of petals present in the cuticle (Liao et al., 2021). In addition, the scent content of the petals decreased at high temperatures. These findings suggest that the cause of high-scent emissions at higher temperature is not an increase in scent production but an increase in vaporization from the cuticle. Here, when the temperature increased from 10 or 15°C to 20°C, the scent emissions of cut tulips increased rapidly (Fig. 3), likely due to the low scent component vaporization from the cuticle at 10 or 15°C, but was accelerated at 20°C. A similar phenomenon is observed in cut carnation flowers (Kishimoto, 2022). This hypothesis may be further supported by comparing the response of the endogenous scent amount in the cuticle and tepal against temperature changes.
The total scent emissions of cut tulips decreased with flower senescence (Figs. 1 and 2). The number of days needed for total emissions at 18°C to exceed those recorded at 23°C differed by cultivar. Specifically, ‘Kung-Fu’, ‘Leo Visser’, ‘Sanne’, and ‘Verona’ were the earliest to show this response. A common feature of these cultivars is the rapid progression of flower senescence at 23°C, as evidenced by abscission and wilting of flower tepals. Previous studies report, for various species of plants, that ethylene induces the scent emission reductions associated with flower senescence (Liu et al., 2017; Schade et al., 2001; Sexton et al., 2005). However, tepal senescence in tulips appears to be induced by intracellular energy depletion rather than ethylene (Azad et al., 2008). Furthermore, in the tulip cultivar ‘Sanne’, a preservation treatment containing an ethylene generator, ethephon, did not affect cut tulip scent emissions (Kishimoto and Watanabe, 2023). Therefore, the senescence-related decrease in scent emissions by tulips may differ from the ethylene-dependent mechanism known for other species of plants.
In several tulip cultivars, scent composition changes were observed primarily before and after flower opening or with floral senescence (Figs. 1 and 2B). In addition, temperature differences also affected scent composition for cultivars, including ‘Leo Visser’ and ‘Strong Gold’ (Fig. 2B), suggesting that the type of tulip scent changes during the anthesis and depending on temperature. For example, Oyama-Okubo and Tsuji (2013) grouped the scent of ‘Monte Carlo’ into “Herbal-Honey” tulips based on the large monoterpene and benzenoid contents that characterized these tulips. Here, however, their scent was dominated only by monoterpenes in the early stages of flower opening, and the scent seemed to fall into a group different from “Herbal-Honey” (Fig. 2B). Comparative analysis using sensory tests is necessary to determine whether these scent composition changes are significant in terms of human olfaction.
The scent composition of ‘Kung-Fu’ and ‘Sanne’ at 23°C on the 1st or 2nd day was clearly different from that at 20°C (Fig. 2B and C1 in Fig. 3C). The tests at these temperatures differed in the state of the flowers on the 1st day, with the flowers at 20°C being less colored and immature (Figs. 1 and 3A). Therefore, the results of the 1st or 2nd day at 20°C may show the scent composition in immature flowers before flower opening or coloring. Furthermore, after the flowers had colored (after 4 days at 20°C in Fig. 3A), there was no noticeable difference in the scent composition of at 20 and 23°C. Such discrepancies were also observed in the scent composition of ‘Ballerina’ on the 8th day at 20 and 23°C, and in the variation patterns of total scent emission in ‘Ballerina’, ‘Kung-Fu’, and ‘Sanne’ at 20 and 23°C. These discrepancies in the results may be explained by differences in flower maturity at the start of the experiment.
The scent emissions of the cut tulips were greater in quantity at higher temperatures under the investigated temperature conditions. However, vase life reduced with increasing temperature. Therefore, it may be difficult to improve both scent emission and vase life under constant high or low-temperature conditions. However, when a low temperature (10 or 15°C) was increased to 20°C, cut flower scent emissions increased remarkably (Fig. 3). This implies that the combination of low temperature and room temperature can control the timing of high-scent emission. For example, if pre-sale cut flowers are kept at the indicated low temperatures, both high-scent emission, and longer vase life are expected for cut flowers placed at room temperature after sale. Low-temperature conditions (10–15°C) during retailing are easily achieved using common display refrigerators. The main harvest season (January to March) for tulip cut flowers in Japan is the coldest season; therefore, active low-temperature management during transportation seems unnecessary. In addition, the artificial control of scent emission through temperature was demonstrated in all three cultivars with different main scent components; therefore, it may be widely applicable to cut tulip cultivars.
Finally, we discuss whether the scent emission upon transfer to room temperature reaches a noticeable scent in the sense of smell. In our previous sensory test (Kishimoto et al., 2018), the scent strength of ‘Ballerina’, ‘Kung-Fu’, and ‘Sanne’ were evaluated by 394 respondents as “very scented”, “scented”, “slightly scented”, or “unscented”. The dotted lines in Figure 3 indicate the threshold level for the quantity of scent emitted for over 70% of the respondents to score flowers as “very scented” or “scented”. Here, we evaluated the intensity of the scent by considering the lower limit as the threshold for detectable scent. As a prerequisite for this evaluation, the scent composition at the time of the sensory test and the scent composition of the cut flowers in this study must be the same. As the scent compositions of these three cultivars, after their transition to 20°C, (Fig. 3C) were similar to those at the time of the sensory test (Kishimoto et al., 2018), the above evaluation is valid. Individual total scent emissions at 10 and 15°C did not reach the lower limit; however, upon transfer to 20°C, it exceeded this limit (Fig. 3B). Therefore, following low-temperature management, scent emissions at room temperature should reach noticeable levels. When comparing pretreatments at 10 and 15°C in ‘Kung-Fu’ and ‘Sanne’, scent emission on the 8th day at 10°C was significantly higher than that at 15°C, and the retention period of noticeable scent was longer. This may be explained by the flowers maturing more slowly at 10°C than at 15°C (Fig. 3A). Therefore, temperatures even lower than 10°C may further delay flower maturity and enhance post-sale noticeable scent and vase life to a greater extent, an issue that deserves further study.
The main scent component of ‘Pretty Woman’ was (E)-β-ocimene (Table 1), but other studies conducted under similar temperature conditions reported 2-hexenal as the major component (Oyama-Okubo and Tsuji, 2013). Several other cultivars, such as ‘Ballerina’ and ‘Strong Gold’, were also found to have scent compositions different from those previously reported (Kishimoto et al., 2018; Oyama-Okubo and Tsuji, 2013). These reports suggest that factors other than temperature and flower opening stage influence cut tulip scent composition. In addition, in Japan, using conservation additives for tulips has become popular (Watanabe, 2020). In the future, it will be important to investigate the combined effects of temperature and factors such as preservatives on scent emission by cut tulips.
In summary, according to the results reported herein, temperature influences the scent emissions of tulip cut flowers, suggesting that scent improvement is possible through temperature management before sale. Cut flowers showed increased scent emission with increasing temperature; concomitantly, vase life was shortened with temperature. Furthermore, at high temperatures, the onset of the scent emission decrease with floral senescence started earlier. At temperatures of 10 or 15°C, scent emission was not high compared to that observed at 20°C; however, the progress of senescence was hindered. Furthermore, the scent emission increased rapidly after flower transfer to 20°C. Therefore, if pre-sale cut tulips are kept at these low temperatures, both high-scent emission and long vase life are expected upon placing them at room temperature after sale. Therefore, temperature control is an effective strategy to be adopted for value addition to cut flowers of tulips based on scent emission optimization.
This research was supported by grants from the Project of the NARO Bio-oriented Technology Research Advancement Institution (Research Program on Development of Innovative Technology; 26103C), Japan. The authors thank Dr. Naomi Oyama-Okubo (Institute of Vegetable and Floriculture Science, NARO, Tsukuba, Japan) for her research advice, and Ms. Mayumi Hongo and Ms. Mariko Hamamura (Institute of Vegetable and Floriculture Science, NARO, Tsukuba, Japan) for their technical assistance. The authors also thank Editage (www.editage.com) for English language editing.