2023 年 92 巻 3 号 p. 323-334
2023 年 92 巻 3 号 p. 323-334
The flower colors and flavonoids of 16 cultivars of Verbena hybrida Groenl. & Rumpler (Verbenaceae) were examined to evaluate the relationship between flower color and flavonoid components. Fifteen anthocyanins {3-O-glucoside, 3,5-di-O-glucoside, 3-O-[6-O-(acetyl)-glucoside], 3-O-[6-O-(malonyl)-glucoside], and 3,5-di-O-[6-O-(acetyl)-glucoside] of delphinidins, cyanidins, and pelargonidins}, seven flavones {7-O-(glucuronide) of apigenin, luteolin, tricetin, and 4'-O-methyl-luteolin, 7-O-[2-O-(glucuronosyl)-glucuronide]-4'-O-(glucuronide) of apigenin, and 7-O-[2-O-(glucuronosyl)-glucuronide] of apigenin and luteolin}, two flavonols {3-O-glucoside and 3-O-[6-O-(acetyl)-glucoside] of kaempferols}, and chlorogenic acid were isolated from the flowers of these cultivars. Their structures were identified using co-HPLC, nuclear magnetic resonance, and mass spectrometry. For the Red to Purple-Violet cultivars, the corresponding color chart names were responsible for the major anthocyanidin types, and the hue values (b*/a*) of these flower colors reflected the anthocyanins concentration. Based on the relationship between the distribution of flavones, flavonols, and chlorogenic acid among cultivars and flower color, we conclude that flavonoids other than anthocyanins and chlorogenic acid make little contribution to flower color. The yellowish red color is thought to be affected by carotenoids.
There are approximately 250 species in the Verbena L. (Verbenaceae) genus (Huxley et al., 1992). Verbena hybrida Groenl. & Rumpler (commom garden Verbena, Verbenaceae) is considered a multiple hybrid involving V. incisa, V. peruviana, V. phlogiflora, and V. teucroides (Huxley et al., 1992). Verbena hybrida is widely cultivated as an annual horticultural plant and is a well-known edible flowers distinguished by colors including Purple-Violet, Purple, Red-Purple, Red, and White with or without contrasting eyes, occasionally marked or striped.
Many reports on the structural analyses of anthocyanins from V. hybrida flowers exist, with derivatives identified including 3,5-di-O-(glucoside)s of delphinidin, cyanidin, and pelargonidin, 3-O-[6-O-(acetyl)-glucoside] of delphinidin, 3-O-[6-O-(malonyl)-glucoside]s of delphinidin, cyanidin, and pelargonidin, 3,5-di-O-[6-O-(acetyl)-glucoside]s of delphinidin, cyanidin, and pelargonidin, 3-O-[6-O-(acetyl)-glucoside]-5-O-glucosides of cyanidin and pelargonidin, 3-O-(glucoside)-5-O-[6-O-(acetyl)-glucoside] of pelargonidin, and 3-O-[6-O-(malonyl)-glucoside]-5-O-[6-O-(acetyl)-glucoside] of pelargonidin (Scott-Moncrieff and Sturgess, 1940; Terahara et al., 1989; Toki et al., 1991a, b, 1995a, b).
In this study, four new floral anthocyanins, 3-O-(glucoside)s of delphinidin and cyanidin, and 3-O-[6-O-(acetyl)-glucoside]s of pelargonidin and cyanidin were discovered from the flowers of V. hibrida. Furthermore, nine flavonoids additional to anthocyanins, 7-O-(glucuronide)s of tricetin, luteolin, apigenin, and 4'-O-methylluteolin, 7-O-[2-O-(glucuronosyl)-glucuronide]s of luteolin and apigenin, 7-O-[2-O-(glucuronosyl)-glucuronide]-4'-O-(glucuronide) of apigenin, 3-O-(glucoside) of kaempferol, and 3-O-[6-O-(acetyl)-glucoside] of kaempferol, and chlorogenic acid, were identified for the first time from the flowers of V. hybrida (Harborne and Baxter, 1999a, b; Veitch and Grayer, 2008, 2011).
Research on V. hybrida flavonoid contents generally focuses on anthocyanin structural analysis using cultivars and strains with darker flower colors (Scott-Moncrieff and Sturgess, 1940; Terahara et al., 1989; Toki et al., 1991a, b, 1995a, b). However, pigment concentration across cultivars is also affected by variations in V. hybrida flower color.
Therefore, using the Obsession series of V. hybrida, which contains the richest variety of flower color, we systematically summarized the relation between flower color and flavonoid distribution.
Verbena hybrida Obsession series seeds were purchased from Takii Seed Co. Ltd. (Kyoto, Japan), Sakata Seed Corporation (Kanagawa, Japan), and M&B Flora & Co. Ltd (Yamanashi, Japan). These seeds were sown in October 2019, and plants grown in a greenhouse at Iwate University. Fresh petals were collected between April and June 2020, dried overnight at 45°C, and stored at −20°C until used for analysis.
Measurements of color and pH of fresh flowersThe color of the fresh flowers for each cultivar were evaluated by comparison with the Royal Horticultural Society (R.H.S.) Colour Chart 5th edition, and their CIE L*a*b* chromaticity values recorded on a CM-700d Spectro Color Meter (Konica-Minolta Co., Ltd., Tokyo, Japan).
The flower color dependency on anthocyanins is sequential, changing in hue from orange to blue, due to the varying ratio of pelargonidin, cyanidin, and delphinidin-type pigments. Therefore, with few exceptions, anthocyanin-derived floral colors are distributed in the first and fourth quadrants of the a*b* coordinates in the CIELab color system. In this study, hue changes in this range are shown as b*/a* values, which can be presented as continuous values, rather than as hue angles, which cannot be shown as continuous values (Hayashi, 1988; Saito et al., 2015). The average was derived from the analysis of three flowers from each cultivar.
The pH values of fresh petal homogenates from each cultivar were measured using a compact pH meter (B-212; Horiba Ltd., Kyoto, Japan). Petals from each cultivar were mashed and the pH of the resulting homogenate measured. This process was repeated three times per cultivar, obtaining the average ± standard error-values (Tatsuzawa et al., 2021).
Quantitative analyses of total pigments in the flowersFor the quantitative study of total anthocyanins and their flavonoids, dried petal (5 mg) of each cultivar was immersed in 0.1% HCl-MeOH (20 mL) at 25°C for 5 h, followed by extraction. The total amount of anthocyanins in these flowers was measured with a UV-Vis spectrophotometer MPS-2450 (Shimadzu, Kyoto, Japan). The main visible absorption maximum for anthocyanins (502–539 nm) and flavonoids (350 nm) for each cultivar was measured. Furthermore, dried petals (10 mg) of each cultivar were immersed in acetone:methanol = 1:1 (v/v) (2 mL) at 25°C for 24 h and extracted for total carotenoid quantitative analysis. Total flower carotenoid content was measured with a UV-Vis spectrophotometer MPS-2450 (Shimadzu).
After detecting the three characteristic absorption maxima of carotenoids at 470, 436, and 410 nm (shoulder) in all cultivars, the absorbance at 450 nm was compared among each cultivar as total carotenoids (Tatsuzawa et al., 2021).
HPLC analysisDried petals (10 mg) from each cultivar were immersed in 5% HOAc (HOAc-H2O, 5:95, v/v, 500 μL) at 25°C for 1 h, followed by pigment extraction for HPLC analysis of flavonoids and chlorogenic acid distribution. Analytical HPLC was performed on an LC 10A system (Shimadzu) using a C18 (4.6 × 250 mm) column (Waters, Milford, MA, USA) at 40°C with a flow rate of 1 mL·min−1. Monitoring was performed at 530 nm for anthocyanins and 350 nm for chlorogenic acid, flavones, and flavonols. The eluant by 5% HOAc-H2O was applied as a linear gradient elution for 40 min from 20 to 85% solvent B (1.5% H3PO4, 20% HOAc, 25% MeCN in H2O) in solvent A (1.5% H3PO4 in H2O) with 5 min of re-equilibration at 20% solvent B (method 1) (Tatsuzawa et al., 2021).
Isolation and purification of acylated anthocyanins, chlorogenic acid, flavones, and flavonolsDried petals of ‘Obsession Lilac’ (ca. 10 g), ‘Obsession Bordeaux’ (ca. 10 g), ‘Obsession Red’ (ca. 9 g), ‘Obsession Crimson with Eye’ (ca. 10 g), ‘Obsession Blue with Eye’ (ca. 10 g), and ‘Obsession Burgundy with Eye’ (ca. 8 g) each were immersed in 5% HOAc-H2O (2 L) at room temperature for 24 h prior to extraction. Each extract was passed through the Diaion HP-20 (Nippon Rensui Co., Tokyo, Japan) columns, on which the pigments were adsorbed. The columns were then thoroughly washed with 5% HOAc-H2O (10 L) and eluted with 5% HOAc-MeOH (500 mL) to recover the pigments. Pigments were separated and purified using paper chromatography (PC) with BAW (n-BuOH/HOAc/H2O, 4:1:2, v/v/v) and concentrated. Separated pigments were further purified using SephadexTM LH-20 (GE Healthcare UK Ltd, Hatfield, UK) column chromatography with 5% HOAc-H2O. The purified pigment 7 (ca. 10 mg from ‘Obsession Burgundy with Eye’), 8 (ca. 8 mg from ‘Obsession Red’), 9 (ca. 21 mg from ‘Obsession Burgundy with Eye’), 10 (ca. 3 mg from ‘Obsession Red’), 11 (ca. 7 mg from ‘Obsession Crimson with Eye’), 12 (ca. 30 mg from ‘Obsession Blue with Eye’), 13 (ca. 22 mg from ‘Obsession Crimson with Eye’), 14 (ca. 78 mg from ‘Obsession Bordeaux’), 15 (ca. 92 mg from ‘Obsession Bordeaux’), 16 (ca. 11 mg from ‘Obsession Lilac’), 17 (ca. 9 mg from ‘Obsession Lilac’), 18 (ca. 2 mg from ‘Obsession Lilac’), 19 (ca. 2 mg from ‘Obsession Lilac’), 20 (ca. 2 mg from ‘Obsession Lilac’), 21 (ca. 3 mg from ‘Obsession Lilac’), 22 (ca. 3 mg from ‘Obsession Bordeaux’), 23 (ca. 22 mg from ‘Obsession Lilac’), 24 (ca. 13 mg from ‘Obsession Blue with Eye’), and 25 (ca. 3 mg from ‘Obsession Bordeaux’) were obtained as powders.
Identification of acid hydrolysates from purified flavonoidsFlavonoids were identified by acid hydrolysis of the purified pigments in 2 N HCl for 2 h at 100°C. Pelargonidin, cyanidin, delphinidin, apigenin, luteolin, tricetin, and kaempferol obtained via acid hydrolysis were identified by comparative HPLC analysis with authentic samples. Analytical HPLC of acid hydrolysates were performed on an LC 20A system (Shimadzu) using a C18 (4.6 × 250 mm) column (Waters) at 40°C with a flow rate of 1 mL·min−1. Monitoring was performed at 530 nm for anthocyanidins and 350 nm for aglycones of flavone and flavonol. The acid hydrolysates in 2 N HCl were separated using a linear gradient elution over 40 min from 20 to 85% solvent B in solvent A with 5 min of re-equilibration at 20% solvent B (see section 2.4., method 1). Glucuronic acid, malonic acid, and acetic acid were eluted by applying an isocratic elution of solvent A for 10 min with monitoring at 210 nm (method 2) (Tatsuzawa, 2020). Thin layer chromatography (TLC) was performed on cellulose-coated plastic sheets (Merck, Darmstadt, Germany), using four mobile phases: BAW (n-BuOH:HOAc:H2O, 4:1:2, v/v/v), EAA (EtOAc:HOAc:H2O, 3:1:1, v/v/v), ETN (EtOH:NH4OH:H2O, 16:1:3, v/v/v), and EFW (EtOAc:HCOOH:H2O, 5:2:1, v/v/v), for sugars with aniline hydrogen phthalate splay reagent (AHP) (Harborne, 1984).
Mass spectrometry and nuclear magnetic resonance (NMR) measurementHigh-resolution fast atom bombardment mass spectra (HR-FABMS) were obtained in positive ion mode with a JMS-700 system (JEOL Ltd., Tokyo, Japan) using glycerol as the matrix. NMR spectra [400 MHz for 1H spectra and 100 MHz for 13C spectra in CD3OD-CF3COOD (9:1) for anthocyanins and in DMSO-d6 for chlorogenic acid, flavones, and flavonols] including 2D (1H-1H COSY, NOESY, 1H-13C HMQC, and 1H-13C HMBC) were recorded on a JNM ECZ-400S (JEOL). Chemical shifts on the δ-scale were recorded using tetramethylsilane as the internal standard. The coupling constants (J) are represented in Hz.
The flower colors of 16 V. hybrida cultivars were classified into five groups Purple-Violet (Purple-Violet N80B~Purple-Violet N81B by R.H.S. Colour Chart with chromaticity values of b*/a* = −1.13–−0.62 by CM-700d), Purple (Purple 76B–Purple N79C, b*/a* = −0.55–−0.35), Red-Purple (Red-Purple 61A–Red-Purple 65A, b*/a* = −0.13–−0.11), Red (Red 38C–Red 55A, b*/a* = 0.12–1.70), and White (White NN155C, b*/a* = −3.29) (Table 1). The pH values of the petal juice extract ranged between 4.87–5.27 with no consistent significant difference between the values for cyanic color cultivars in this study (Table 1). Therefore, the impact of pH on flower color variation was deemed low.
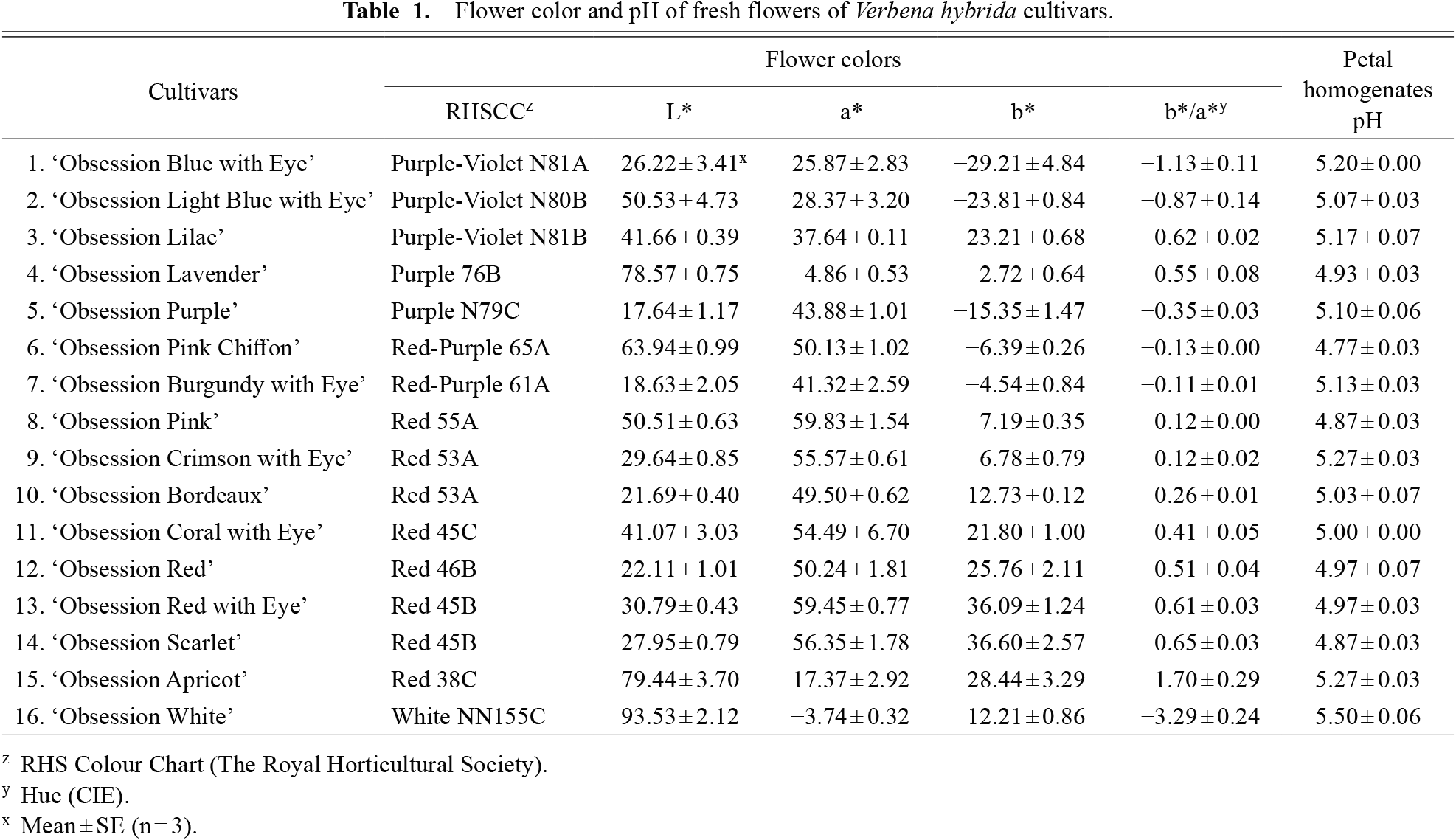
Flower color and pH of fresh flowers of Verbena hybrida cultivars.
Comparing authentic samples, six peaks (numbered 1–6) in the HPLC analysis (530 nm) detected between 8.0 and 15.0 min of retention time were easily identified as delphinidin 3,5-di-O-glucoside, cyanidin 3,5-di-O-glucoside, pelargonidin 3,5-di-O-glucoside, delphinidin 3-O-glucoside, cyanidin 3-O-glucoside, and pelargonidin 3-O-glucoside, respectively (Table 2; Fig. 1) (Tatsuzawa and Shinoda, 2005). The anthocyanins 1, 2, 3, and 6 are reported in flowers of V. hybrida (Table S1, Harborne and Baxter, 1999b).

Distribution of anthocyanin in the flowers of Verbena hybrida cultivars.

Anthocyanins from the flowers of Verbena hybrida.
HR-FABMS and NMR evaluated nine peaks (numbered 7–15) in the HPLC analysis detected at 530 nm with between 15.0 and 30.0 min of retention time (Table 2). Moreover, HR-FABMS and NMR identified one peak of chlorogenic acid (16), seven peaks of flavones (17–21, 23, and 24), and two peaks of flavonols (22 and 25) in the HPLC analysis at 350 nm (Table 3).
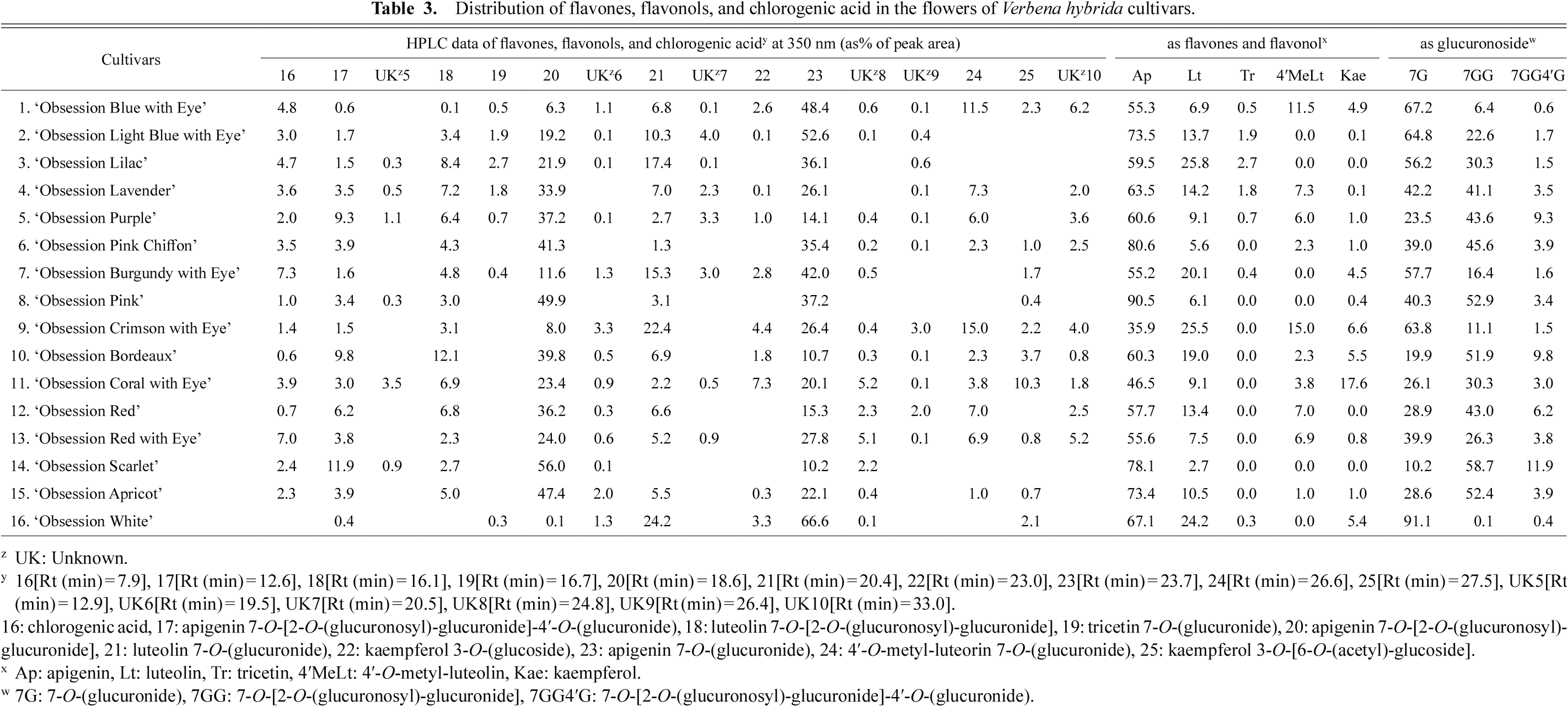
Distribution of flavones, flavonols, and chlorogenic acid in the flowers of Verbena hybrida cultivars.
Acid hydrolysis of anthocyanins 7, 8, and 10 yielded delphinidin [Rt (min) 20.9 (method 1)], cyanidin [Rt (min) 25.4 (method 1)], and pelargonidin [Rt (min) 30.8 (method 1)], respectively, as anthocyanidin. Then, β-glucose [TLC:(Rf-values) BAW 0.23, EAA 0.37, ETN 0.62, EFW 0.41, color with AHP: Brown] and malonic acid [Rt (min) 3.5 (method 2)] were obtained as hydrolysates from the anthocyanins 7, 8, and 10. The molecular ions [M]+ of anthocyanins 7, 8, and 10 were 551, 535, and 519 m/z in FAB mass spectra, which agreed with the mass estimates for C24H23O15, C24H23O14, and C24H23O13, respectively. The elemental composition of these anthocyanins was confirmed using high-resolution FAB mass spectra; calc. for C24H23O15 requires: 551.1037, found: 551.1053 for anthocyanin 7, calc. for C24H23O14 requires: 535.1088, found: 535.1099 for anthocyanin 8, and calc. for C24H23O13 requires: 519.1139, found: 519.1139 for anthocyanin 10. According to these values, anthocyanins 7, 8, and 10 were composed of delphinidin, glucose, and malonic acid in 7, cyanidin, glucose, and malonic acid in 8, and pelargonidin, glucose, and malonic acid in 10, respectively.
In the 1H NMR spectra, the chemical shifts of five aromatic protons from the delphinidin moiety in anthocyanin 7, six aromatic protons from cyanidin moiety in anthocyanin 8, and seven aromatic protons from the pelargonidin moiety in anthocyanin 10, with their coupling constants, are assigned as listed in Table S2. The sugar moieties of anthocyanins 7, 8, and 10 have proton chemical shifts in the region δ 5.32–3.43, with anomeric proton resonances at δ 5.32 (d, J = 7.8 Hz, anthocyanin 7), δ 5.29 (d, J = 7.8 Hz, anthocyanin 8), and δ 5.27 (d, J = 7.8 Hz, anthocyanin 10).
These sugars are considered β-pyranose forms based on the observed coupling constants, (Table S2). The linkage and/or position of the attachment of the sugar and acyl group were determined based on 2D COSY and NOESY experiments. By application of the NOESY experiment, NOEs between the H-1 of glucose and H-4 (δ 8.87) of delphinidin (anthocyanin 7), between the H-1 of glucose and H-4 (δ 8.96) of cyanidin (anthocyanin 8), and between the H-1 of glucose and H-4 (δ 9.02) of pelargonidin (anthocyanin 10) were observed, respectively, indicating glycosylation of the C-3 anthocyanidin hydroxy group with glucose. Two characteristic downfield shifted proton signals were assigned to the methylene protons of glucose (δ 4.31 and 4.58, H-6a and b of anthocyanin 7, δ 4.30 and 4.57, H-6a and b of anthocyanin 8, and δ 4.30 and 4.57, H-6a and b of anthocyanin 10), indicating acylation of the glucose C-6 OH. In the HMBC spectrum of anthocyanin 7, correlation between the anomeric proton of glucose and the C-3 carbon (δ 145.8) of delphinidin then methylene protons of glucose and the COOH carbon (δ 168.9) of malonic acid were observed, thus establishing glycosylation at the delphinidin C-3 OH and acylation at the glucose C-6 OH. Consequently, the structure of anthocyanin 7 was determined as delphinidin 3-O-[6-O-(malonyl)-β-glucopyranoside] (Fig. 1). Furthermore, by comparing 1H NMR of anthocyanins 7, 8, and 10, the structures of 8 and 10 were identified as cyanidin 3-O-[6-O-(malonyl)-β-glucopyranoside] and pelargonidin 3-O-[6-O-(malonyl)-β-glucopyranoside], respectively (Fig. 1). Anthocyanins 7, 8, and 10 are pigments reported in V. hybrida (Table S1, Harborne and Baxter, 1999b).
2.2. Anthocyanins 9, 11, and 13The acid hydrolysis of anthocyanins 9, 11, and 13 yielded delphinidin, cyanidin, and pelargonidin, respectively, as anthocyanidin. Then, β-glucose and acetic acid [Rt (min) 3.7 (method 2)] were obtained as hydrolysates from the anthocyanins 9, 11, and 13.
The FAB mass spectra of anthocyanins 9, 11, and 13 gave their molecular ions [M]+ at 507, 491, and 475 m/z, agreeing with the masses calculated for C23H23O13, C23H23O12, and C23H23O11, respectively. The elemental composition of these anthocyanins was confirmed by measuring their high-resolution FAB mass spectra; calc. for C23H23O13 requires: 507.1139, found: 507.1120 for anthocyanin 9, calc. for C23H23O12 requires: 491.1190, found: 491.1184 for anthocyanin 11, and calc. for C23H23O11 requires: 475.1240, found: 475.1244 for anthocyanin 13. According to these results, anthocyanins 9, 11, and 13 are composed of delphinidin, glucose, and acetic acid, then cyanidin, glucose, and acetic acid, and finally pelargonidin, glucose, and acetic acid, respectively.
The 1H NMR spectra of anthocyanins 9, 11, and 13 were comparable to those of anthocyanins 7, 8, and 10, except that the signals of -CH2- of malonic acid (δ 3.43 of 7, δ 3.42 of 8, and δ 3.41 of 10) were replaced by acetic acid -CH3 signals (δ 2.04 of 9, δ 2.04 of 11, and δ 2.06 of 13) (Table S2). Consequently, the structures of anthocyanins 9, 11, and 13 were identified as delphinidin 3-O-[6-O-(acetyl)-β-glucopyranoside], cyanidin 3-O-[6-O-(acetyl)-β-glucopyranoside], and pelargonidin 3-O-[6-O-(acetyl)-β-glucopyranoside], respectively (Fig. 1). Anthocyanin 9 is previously reported in V. hybrida (Table S1, Harborne and Baxter, 1999b). However, anthocyanins 11 and 13 were identified for the first time in V. hybrida (Table S1, Harborne and Baxter, 1999b).
2.3. Anthocyanins 12, 14, and 15The acid hydrolysis of anthocyanins 12, 14, and 15 yielded delphinidin, cyanidin, and pelargonidin, respectively, as anthocyanidin. Then, β-glucose and acetic acid were obtained as hydrolysates from the anthocyanins 12, 14, and 15.
The FAB mass spectra of anthocyanins 12, 14, and 15 gave their molecular ions [M]+ at 711, 695, and 679 m/z, agreeing with masses calculated for C31H35O19, C31H35O18, and C31H35O17, respectively. The elemental composition of these anthocyanins was confirmed by measuring their high-resolution FAB mass spectra; calc. for C31H35O19 requires: 711.1773, found: 711.1762 for anthocyanin 12, calc. for C31H35O18 requires: 695.1823, found: 695.1848 for anthocyanin 14, and calc. for C31H35O17 requires: 679.1874, found: 679.1862 for anthocyanin 15. According to these results, anthocyanin 12 is composed of delphinidin with two molecules each of glucose and acetic acid, anthocyanin 14 is composed of cyanidin with two molecules each of glucose and acetic acid, and anthocyanin 15 is composed of pelargonidin with two molecules each of glucose and acetic acid.
The 1H NMR spectra of anthocyanins 12, 14, and 15 were comparable to those of anthocyanins 9, 11, and 13, except for the additional signals each of glucose and acetic acid as shown in Table S2. Through the NOESY experiment, NOEs between the H-1 of the additional glucose and H-6 of the anthocyanidin skeletons of 12, 14, and 15 were observed. Additionally, two characteristic downfield shifted proton signals were assigned to the methylene protons of the additional glucose (δ 4.31 and 4.44, H-6a and b of anthocyanin 12, δ 4.32 and 4.44, H-6a and b of anthocyanin 14, and δ 4.31 and 4.44, H-6a and b of anthocyanin 15), indicating acylation at the C-6 OH of the additional glucose. In the HMBC spectrum of anthocyanins 14 and 15, a correlation between the methylene protons of the additional glucose and COOH carbon (δ 172.9 in 14 and 172.9 in 15) of acetic acid was observed, establishing acetylation at C-6 OH of the additional glucose.
Consequently, the structures of anthocyanins 12, 14, and 15 were determined as delphinidin 3,5-di-O-[6-O-(acetyl)-β-glucopyranoside], cyanidin 3,5-di-O-[6-O-(acetyl)-β-glucopyranoside], and pelargonidin 3,5-di-O-[6-O-(acetyl)-β-glucopyranoside], respectively (Fig. 1). Anthocyanins 12, 14, and 15 are previously reported pigments in V. hybrida (Table S1, Harborne and Baxter, 1999b).
2.4. Flavones 17, 20, and 23Acid hydrolysis of flavones 17, 20, and 23 yielded apigenin and glucuronic acid [Rt(min) 3.2 (method 2)] as hydrolysates of the three flavones.
The FAB mass spectra of flavones 17, 20, and 23 yielded molecular ions [M+H]+ at 799, 623, and 447 m/z, agreeing with masses calculated for C33H35O23, C27H27O17, and C21H19O11, respectively. The elemental composition of these flavones was confirmed by measuring their high-resolution FAB mass spectra; calc. for C33H35O23 requires: 799.1569, found: 799.1561 for flavone 17, calc. for C27H27O17 requires: 623.1248, found: 623.1257 for flavone 20, and calc. for C21H19O11 requires: 447.0927, found: 447.0930 for flavone 23. According to these results, flavone 17 is composed of apigenin with three molecules of glucuronic acid, flavone 20 is composed of apigenin with two molecules of glucuronic acid, and flavone 23 is composed of apigenin with one molecule of glucuronic acid.
The 1H and 13C NMR spectra of flavone 17 revealed seven aromatic proton signals from apigenin (Table S3). Three anomeric proton signals from glucuronic acids I, II, and III were observed at δ 5.34 (d, J = 7.0 Hz), 4.55 (d, J = 7.8 Hz), and 5.18 (d, J = 7.0 Hz), respectively.
NOESY and HMBC studies validated the assignment of glucuronic acids I, II, and III. The NOEs between the H-1 of glucuronic acids I, II, and III, with H-6 and 8 of apigenin, H-2 of glucuronic acid I, and both H-3' and 5' of apigenin were observed. Furthermore, HMBCs were discovered between the H-1 of glucuronic acids I, II, and III and the C-7 of apigenin, C-2 of glucuronic acid I, and C-4' of apigenin. Therefore, flavone 17 was determined as apigenin 7-O-[2-O-(β-glucuropyranosyl(II))-β-glucuropyranoside(I)]-4'-O-(β-glucuropyranoside(III)) (Fig. 2), a previously identified flavone (Stochmal et al., 2001). However, this is the first time that flavone 17 was identified in V. hybrida flowers (Harborne and Baxter, 1999a).

Flavones from the flowers of Verbena hybrida.
The 1H and 13C NMR spectra of flavone 20 were similar to those of flavone 17, but for the absence of glucuronic acid III (Table S3). All proton and carbon chemical shifts for flavone 20 were assigned using the same method as used for flavone 17. The link between the flavone and glucuronic acid I moieties and the glucuronic acid I and II moieties were confirmed by NOESY and HMBC spectra. Therefore, flavone 20 was identified as apigenin 7-O-[2-O-(β-glucuropyranosyl(II))-β-glucuropyranoside(I)] (Fig. 2). Flavone 20 is previously reported in other species of the same genus Verbena (Gibitz-Eisath et al., 2020; Harborne and Baxter, 1999a).
The 1H and 13C NMR spectra of flavone 23 were similar to those of flavone 17, except for the absence of glucuronic acid II and III (Table S3). All proton and carbon chemical shifts for flavone 23 were assigned using the same method as that used for flavones 17 and 20. The link between the flavone and glucuronic acid I moieties was confirmed by NOESY and HMBC spectra. Therefore, flavone 23 was identified as apigenin 7-O-(β-glucuropyranoside(I)) (Fig. 2). Flavone 23 is a previously reported pigment in other species of the same genus, Verbena (Gibitz-Eisath et al., 2020; Harborne and Baxter, 1999a).
2.5. Flavones 18 and 21The acid hydrolysis of flavones 18 and 21 yielded luteolin and glucuronic acid as hydrolysates from the two flavones.
The FAB mass spectra of flavones 18, and 21 yielded molecular ions [M+H]+ at 639 and 463 m/z, agreeing with the mass calculated for C27H27O18 and C21H19O12, respectively. The elemental composition of these flavones was confirmed by measuring their high-resolution FAB mass spectra; calc. for C27H27O18 requires: 639.1197, found: 639.1186 for flavone 18 and calc. for C21H19O12 requires: 463.0877, found: 463.0889 for flavone 21. According to these results, flavone 18 is composed of luteolin with two molecules of glucuronic acid and flavone 21 is composed of luteolin with one molecule of glucuronic acid.
The 1H and 13C NMR spectra revealed that the chemical shifts of flavones 18 and 21 were identical to those of flavones 20 and 23, respectively, except for the presence of luteolin but not apigenin (Table S3). Therefore, flavones 18 and 21 were identified as luteolin 7-O-[2-O-(β-glucuropyranosyl(II))-β-glucuropyranoside(I)] and luteolin 7-O-(β-glucuropyranoside(I)), respectively (Fig. 2). Flavones 18 and 21 are previously reported in other species of the same genus Verbena (Gibitz-Eisath et al., 2020; Harborne and Baxter, 1999a).
2.6. Flavone 19The acid hydrolysis of flavone 19 yielded tricetin and glucuronic acid as hydrolysates.
The FAB mass spectra of flavone 19 yielded its molecular ion [M+H]+ at 479 m/z, agreeing with the mass calculated for C21H19O13. The elemental composition of this flavone was confirmed by measuring its high-resolution FAB mass spectra; the calc. for C21H19O13 requires: 479.0826, found: 479.0845. According to these results, flavone 19 is composed of tricetin with one molecule of glucuronic acid.
The 1H and 13C NMR spectra revealed identical chemical shifts for flavone 19 to flavone 23, except for the presence of tricetin but not apigenin (Table S3). Therefore, 19 was identified as tricetin 7-O-(β-glucuropyranoside(I)) (Fig. 2), a previously identified flavone (Wang et al., 2015). However, flavone 19 was identified for the first time in Verbena hybrida flowers (Harborne and Baxter, 1999a).
2.7. Flavone 24The acid hydrolysis of flavone 24 yielded 4'-methyl-luteolin and glucuronic acid as hydrolysates.
The FAB mass spectra of flavone 24 yielded the molecular ion [M+H]+ at 477 m/z, agreeing with the mass calculated for C22H21O12. The elemental composition of this flavone was confirmed by measuring its high-resolution FAB mass spectra; calc. for C22H21O12 requires: 477.1033, found: 477.1043. According to these results, flavone 24 is composed of 4'-methyl-luteolin with one molecule of glucuronic acid.
The 1H and 13C NMR spectra revealed that the chemical shifts of flavone 24 were identical to those of flavone 21, except for the presence of 4'-methyl-luteolin but not luteolin (Table S3).
Therefore, flavone 24 was identified as 4'-methyl-luteolin (diosmetin) 7-O-(β-glucuropyranoside(I)) (Fig. 2), a previously known flavone (Archana et al., 2013). However, flavone 24 was identified for the first time in V. hybrida (Harborne and Baxter, 1999a).
2.8. Flavonols 22 and 25The acid hydrolysis of flavonols 22 and 25 yielded kaempferol and glucose, and kaempferol, glucose, and acetic acid, respectively, obtained as hydrolysates.
The FAB mass spectra of flavonols 22 and 25 afforded their molecular ions [M+H]+ at 449 and 491 m/z, agreeing with the mass calculated for C21H21O11 and C23H23O12, respectively. The elemental composition of these flavonols was confirmed by measuring their high-resolution FAB mass spectra; calc. for C21H21O11 requires: 449.1084, found: 449.1084 for flavonol 22 and calc. for C23H23O12 requires: 491.1190, found: 491.1187 for flavone 25. These values indicate that flavonol 22 is composed of kaempferol with one glucose molecule and flavonol 25 is composed of kaempferol with one molecule of glucose and acetic acid.
The 1H and 13C NMR spectra revealed that the chemical shifts of flavone 25 were identical to those of anthocyanin 13, except for the presence of kaempferol but not pelargonidin (Tables S2 and S3). Therefore, flavone 25 was identified as kaempferol 3-O-[6-O-(acetyl)-β-glucopyranoside] (Fig. 3), a previously identified flavonol (Slimestad et al., 1995). However, flavonol 25 was identified for the first time in V. hybrida (Harborne and Baxter, 1999a).
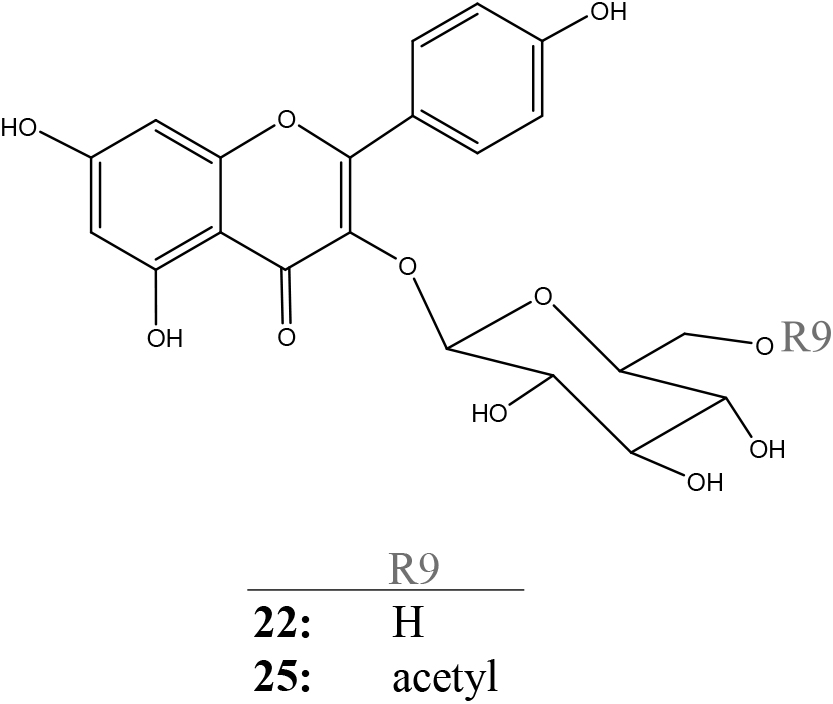
Flavonols from the flowers of Verbena hybrida.
The 1H and 13C NMR spectra of flavonol 22 were similar to those of flavonol and glucose moieties in flavonol 25, but for the absence of the acetic acid (Table S3). All proton and carbon chemical shifts for flavonol 22 were assigned using the same method as that used for flavonol 25. The link between the flavonol and glucose moieties was confirmed by NOESY and HMBC spectra. Therefore, flavonol 22 was determined as kaempferol 3-O-(β-glucopyranoside) (Fig. 3), a previously known flavonol (Slimestad et al., 1995). However, flavonol 22 was identified for the first time in V. hybrida (Harborne and Baxter, 1999a).
2.9. Clorogenic acid (16)Structure 16 was identified as chlorogenic acid by direct comparison of its HPLC [Rt(min) 7.9], high-resolution FAB mass spectra {[M+H]+; calc. for C16H19O9 requires: 355.1029, found: 355.1041}, and 1H and 13C NMR spectra (Table S4) with commercial standard (Wako Pure Chemical Industries, Ltd., Osaka, Japan).
3. Distribution of flavonoids and flower colors in 16 cultivars of the Obsession series of Verbena hybridaThe R.H.S. Colour Chart divided the 16 cultivars of the Verbena hybrida Obsession series into five groups (Purple-Violet, Purple, Red-Purple, Red, and White) based on their flower colors (Table 1).
The hue values (b*/a*) of the Purple-Violet group (‘Obsession Blue with Eye’, ‘Obsession Light Blue with Eye’, and ‘Obsession Lilac’) were −1.13–−0.62 on the CM-700d Spectro color meter, and the pH of their petal homogenates were 5.07–5.20 (Table 1). According to the HPLC study on anthocyanin composition, the major anthocyanins ‘Obsession Blue with Eye’ and ‘Obsession Light Blue with Eye’ are delphinidin 3,5-di-O-[6-O-(acetyl)-glucoside] (12), and ‘Obsession Lilac’ was determined as delphinidin 3-O-[6-O-(acetyl)-glucoside (9) (Table 2). When the proportions of the anthocyanidin skeletons, including other minor anthocyanins, were compared in all three cultivars, delphinidin was overwhelmingly higher than cyanidin and pelargonidin (Table 2). Furthermore, anthocyanins have a higher proportion of acetyl groups than malonyl groups. The glycoside types of anthocyanins were ‘Obsession Blue with Eye’ and ‘Obsession Light Blue with Eye’, which had more 3,5-diglycoside, and ‘Obsession Lilac’, which had more 3-glycoside, but the effect on flower color was found to be small. Thus, the flower color of this group is heavily influenced by delphinidin, which has the highest proportion of anthocyanidins (Fig. 4).

Distribution of flower colors in Verbena hybrida.
The hue values (b*/a*) of the Purple group (‘Obsession Lavender’ and ‘Obsession Purple’) were −0.55 and −0.35 on the CM-700d Spectro color meter, and the pH of their petal homogenates was 4.93 and 5.10 (Table 1). According to HPLC analysis on anthocyanin composition, the major anthocyanin of ‘Obsession Lavender’ was delphinidin 3,5-di-O-[6-O-(acetyl)-glucoside] (12), the major anthocyanidin skeleton was delphinidin, the major glycoside type was 3,5-diglycoside, and the major acyl group was acetyl group, as were ‘Obsession Blue with Eye’ and ‘Obsession Light Blue with Eye’ belonging to the Purple-Violet group (Table 2).
The semi-major anthocyanins of ‘Obsession Purple’ were identified as delphinidin 3-O-(malonyl)-glucoside (7) and delphinidin 3-O-[6-O-(acetyl)-glucoside] (9), according to the HPLC study of anthocyanin composition (Table 2). When the proportions of the anthocyanidin skeletons, including other minor anthocyanins, were compared in these two cultivars, delphinidin was higher than cyanidin and pelargonidin (Table 2). However, in ‘Obsession Purple’, the proportion of cyanidin was found to be higher than in ‘Obsession Lavender’. Furthermore, ‘Obsession Lavender’ has the same type of acyl groups connected to anthocyanins and glycoside types of anthocyanin as ‘Obsession Lilac’, both belonging to the Purple-Violet group (Fig. 4).
The hue values (b*/a*) of the Red-Purple group (‘Obsession Pink Chiffon’ and ‘Obsession Burgundy with Eye’) were −0.13 and −0.11 on the CM-700d Spectro color meter, and the pH of their petal homogenates was 4.77 and 5.13 (Table 1). According to HPLC analysis on anthocyanin composition, the major anthocyanins of ‘Obsession Pink Chiffon’ was pelargonidin 3,5-di-O-[6-O-(acetyl)-glucoside] (15), the major anthocyanidin skeleton was pelargonidin, the major glycoside type was 3,5-diglycoside, and the major acyl group was acetyl group (Table 2). The semi-major anthocyanins of ‘Obsession Burgundy with Eye’ were identified as delphinidin 3-O-(malonyl)-glucoside (7) and delphinidin 3-O-[6-O-(acetyl)-glucoside] (9), according to the results of HPLC analysis of anthocyanin composition (Table 2). When the proportions of the anthocyanidin skeletons, including other minor anthocyanins, were compared, it was found that delphinidin was higher than cyanidin and pelargonidin in ‘Obsession Burgundy with Eye’ as were ‘Obsession Purple’, belonging to the Purple group (Table 2). Furthermore, the types of acyl groups attached to anthocyanins and the glycoside type of anthocyanins are very similar to those of ‘Obsession Purple’ (Fig. 4).
The hue values (b*/a*) of the Red group (‘Obsession Pink’, ‘Obsession Crimson with Eye’, ‘Obsession Bordeaux’, ‘Obsession Coral with Eye’, ‘Obsession Red’, ‘Obsession Red with Eye’, ‘Obsession Scarlet’ and ‘Obsession Apricot’) were 0.12–1.70 on the CM-700d Spectro color meter, and their pH of their petal homogenates was 4.87 and 5.27 (Table 1). According to HPLC analysis on anthocyanin composition, the distribution of major and semi-major anthocyanins in this group consisted of various combinations of cyanidin 3-O-(malonyl)-glucoside (8), pelargonidin 3-O-(malonyl)-glucoside (10), cyanidin 3-O-[6-O-(acetyl)-glucoside] (11), pelargonidin 3-O-[6-O-(acetyl)-glucoside] (13), cyanidin 3,5-di-O-[6-O-(acetyl)-glucoside] (14), and pelargonidin 3,5-di-O-[6-O-(acetyl)-glucoside] (15). As there was no regularity in the type of acyl groups attached to anthocyanins and the glycoside types of anthocyanins, the correlation with flower color was low. When the proportions of the anthocyanidin skeletons, including other minor anthocyanins, were compared, it was discovered that pelargonidin was much higher than cyanidin as were ‘Obsession Pink Chiffon’ belonging to the Red-Purple group in ‘Obsession Pink’, ‘Obsession Coral with Eye’, ‘Obsession Red’, ‘Obsession Red with Eye’, ‘Obsession Scarlet’, and ‘Obsession Apricot’ (Table 2). When comparing the proportions of the anthocyanidin skeletons, including other minor anthocyanins, ‘Obsession Crimson with Eye’ and ‘Obsession Bordeaux’ it was discovered that cyanidin was higher than pelargonidin (Table 2; Fig. 4).
When comparing the absorption maximum wavelengths and their λvis maxima of crude extracted anthocyanins by 0.1% HCl-MeOH in all cultivars, the λvis maxima of the six cultivars of the Purple-Violet and Purple groups, and ‘Obsession Burgandy with Eye’ (Red-Purple group) were 538–539 nm (Table 4). The primary anthocyanidin in these cultivars is delphinidin. The absorbance of λvis maxima was higher in ‘Obsession Purple’ and ‘Obsession Burgandy with Eye’ among these six cultivars than in other cultivars (Table 4), indicating that the concentration of anthocyanins was high, and the redness of the flower color was increased, similar to Matthiola incana (Tatsuzawa et al., 2012). On the contrary, in ‘Obsession Lavender’, it was considered that the bluish effect was increased due to the low concentration of anthocyanins (Tatsuzawa et al., 2012) (Fig. 4).
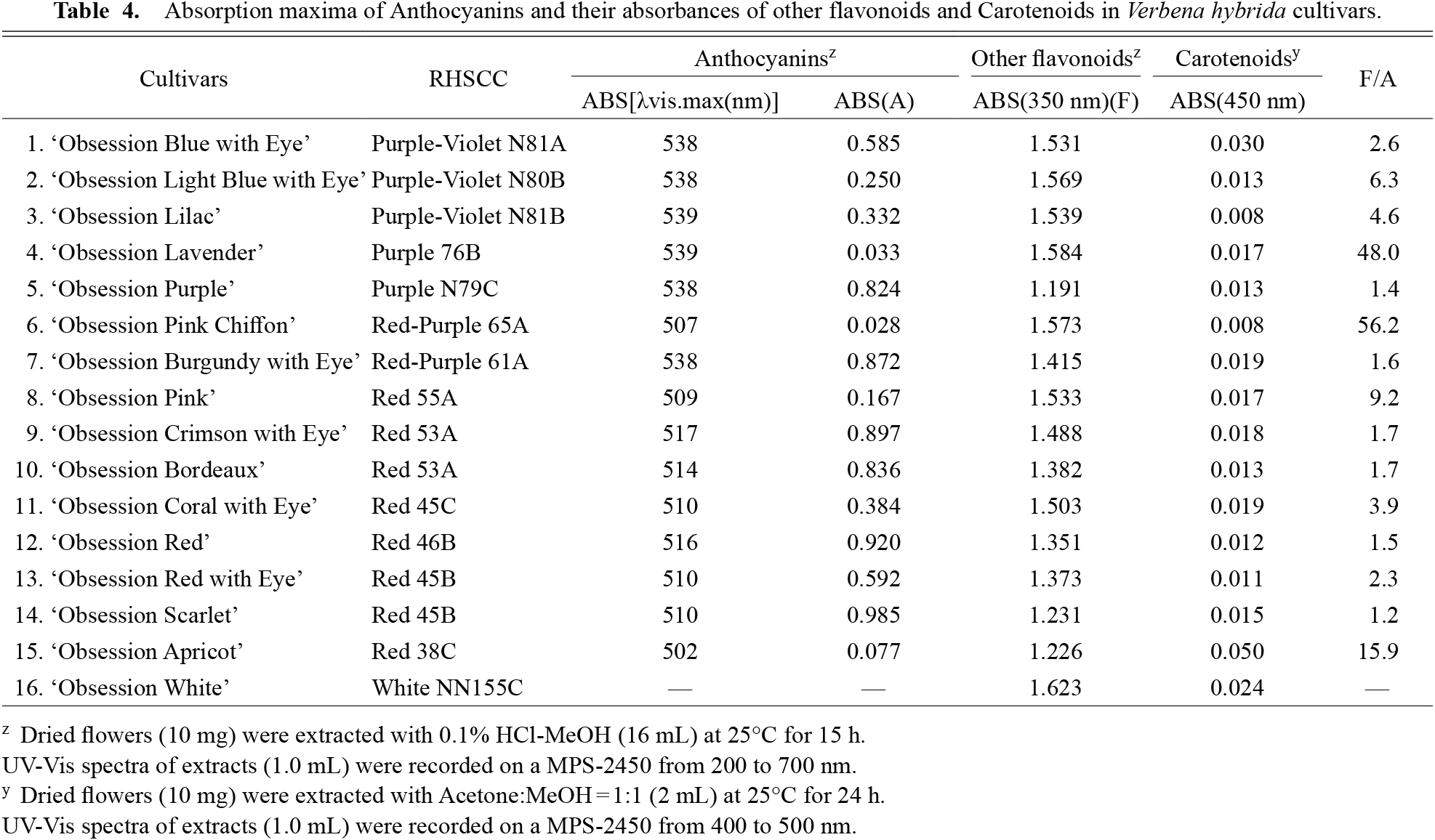
Absorption maxima of Anthocyanins and their absorbances of other flavonoids and Carotenoids in Verbena hybrida cultivars.
The λvis maxima of the eight cultivars of the Red group and ‘Obsession Pink Chiffon’ (Red-Purple group) were 502–517 nm (Table 4). Pelargonidin or cyanidin is the main anthocyanidin in these nine cultivars. Among the two cultivars having cyanidin as the main anthocyanidin, ‘Obsession Crimson with Eye’ and ‘Obsession Bordeaux’ tended to have a bluing effect due to the low anthocyanin concentration (Table 4) (Tatsuzawa et al., 2012) and was classified in the Red-Purple group. The remaining seven cultivars were from the Red group and contain pelargonidin as the primary anthocyanidin (Table 2). Among these cultivars, ‘Obsession Apricot’ had a low concentration of anthocyanins, tending to have more carotenoids than other cultivars (Table 4), this was considered to result in a yellowish effect (Tatsuzawa et al., 2012) (Fig. 4).
In the White group, no anthocyanins were detected (Tables 2 and 4). Furthermore, as the distribution and amount of the main flavones and flavonols did not differ significantly from that of the other 15 cultivars, it is assumed that flavones and flavonols have little influence on cyanic flower color expression in V. hybrida.