2023 年 92 巻 3 号 p. 245-254
2023 年 92 巻 3 号 p. 245-254
Shoot regeneration experiments from growth-point-derived callus were conducted to improve apple genome editing techniques. The plant material studied was in vitro maintained shoots of ‘Fuji’. In the first step, a procedure for shoot formation from apple callus was established. After using the two earlier reported cases’ experimental procedures and media compositions, we investigated the effect of media variations in callus induction, callus multiplication, and shoot induction from the meristem. The procedure that yielded a higher shoot regeneration rate with meristem-derived callus involved shoot multiplication with 1001 medium followed by the Caboni’s callus induction medium, then without callus propagation on liquid medium, and then either Caboni’s or Saito-Suzuki’s shoot induction medium. In this experiment, axillary buds may remain as the shoot apexes were excised at 1 mm. In the next experiment, shoot apexes were excised at 0.5 mm to completely eliminate the axillary bud. Then treatment in the dark was added to the procedure to further improve shoot regeneration rates for the meristem-derived callus. Shoot multiplication medium and shoot induction medium procedures were conducted under dark conditions. This yielded the following optimal procedure: excising meristems from chlorosis shoots after 2–3 months of dark treatment in shoot multiplication medium on 1001; placing the excised meristems in Carboni’s callus induction medium in the dark for 20 days; then transferring the formed callus Saito-Suzuki’s shoot induction medium with incubation in the dark for at least 2 weeks. The shoot regeneration rates of calli treated in the dark for 6 weeks with shoot induction medium reached 68%. Relative to previous reports, this value is considered high for shoot regeneration from calli in apple cultivars.
Introducing desired traits into apple cultivars without negatively altering genetic composition by crossing is difficult as apples are self-incompatible and highly heterozygous, with inbreeding depression. Therefore, improved genetic modification techniques for apple breeding are essential. A transformation system using Agrobacterium is established for apples (De Bondt et al., 1996; Ito et al., 1997; James et al., 1989; Komori et al., 1997; Li et al., 2011; Maximova et al., 1998; Sriskandarajah et al., 1994). However, transgenic plant cultivation is virtually impossible in Japan. It is expected that transgenic plant distribution and use will continue to face difficulties, but insertions and deletions of a few nucleotides are not subject to the Cartagena Act. Therefore, certain genome-edited individuals are not treated as transgenic transformants (Ministry of the Environment, 2019). To modify genes freely without violating domestic law, it is necessary to enable genome editing by direct gene transfection without using the Agrobacterium method. Unlike the conventional Agrobacterium method, genome editing technology can modify genes without traces of gene transfer (Ezura and Osawa, 2013). Genome editing technology for apples was developed recently (Nishitani et al., 2016), but the technology is editing via a transformation system in which CRISPR/Cas9 sequences are introduced into Agrobacterium. Achieving genome editing by transient expression is necessary to introduce a ribonucleoprotein complex (RNP) such as CRISPR/Cas9 into cells using particle bombardment or electroporation to induce mutations. Also, the efficient regeneration of individuals from cells is necessary. An example of successful and efficient shoot regeneration from fruit tree cells is the citrus shuttle callus system (Hidaka, 1990; Hidaka and Omura, 1989). The shuttle callus system is an experimental system in which the callus is preserved, and the shoots or embryos are obtained simply by transferring the callus to the regeneration medium. Adopting this system for apple cultivation necessitates establishment of a callus-based regeneration system. In apples, shoot regeneration from meristem-derived callus (Caboni et al., 2000; Saito and Suzuki, 1999b) and from callus derived from nucellus cells (Saito et al., 1989) was achieved around 2000. Nevertheless, few successful cases of regeneration from callus (Liu et al., 1994; Rugini and Muganu, 1998) are since reported. Earlier studies used cultivars such as ‘Fuji’, ‘Gala’, ‘Golden Delicious’, ‘Jonagold’, and ‘McIntosh’, but did not reach a level of practical technology due to low shoot regeneration rates and process complexity. The ‘Fuji’ apple cultivar was used in this experiment as it is the most widely produced cultivar in Japan, and the world, but is challenging as it is not especially amenable to shoot regeneration experiments.
For this experiment, we selected a combination of effective media for shoot regeneration from meristem-derived callus from the using media developed by Saito and Suzuki (1999b) and of Caboni et al. (2000). Furthermore, we improved callus shoot regeneration rate by evaluating culture procedures such as using a dark treatment period.
‘Fuji’ branches harvested from the tree in a field on May 9, 2018, were sprouted in the laboratory. Shoots were then placed in vitro (primary culture) with a primary culture medium composition of MS basic medium (Murashige and Skoog, 1962) containing 1/2 inorganic salts with 0.1 mg·L−1 benzylaminopurine (BAP), 3% sucrose, and 0.65% Bacto Agar. Thereafter, the plants were maintained in subculture. Subculture was conducted monthly with 1001 medium (Ito et al., 1997; Komori et al., 1997), of which the composition was MS basic medium with 0.1 mg·L−1 indoleacetic acid (IBA), 1.0 mg·L−1 BAP, 3% sucrose, and 0.68% Bacto agar. In the subculture, three shoots were planted per culture bottle. Shoots were then incubated at 25°C with a 16 h day length.
The callus and shoot induction procedures are listed in Table 1, along with the medium compositions. At 6.5 months after primary culture, shoots subcultured on 1001 medium were transferred to the shoot multiplication medium developed by Caboni et al. (2000) (abbreviated hereinafter as Caboni’s medium), 1001 medium, and 1000 medium (Table 1). Cuttings of the meristems from shoots grown in 1001 medium were taken at 7.5 months after primary culture. Meristems were cut from shoots subcultured on the shoot multiplication medium of Caboni or 1000 after an additional 1–3 months of subculture. The meristems were excised from shoots 1 month after subculture and were cut at 1.0 mm lengths using an injection needle (NANOPASS 34G; Terumo Corp., Tokyo, Japan) under a stereomicroscope on a clean bench. The excised meristems were placed on callus two types of induction media: Caboni’s and the medium developed by Saito and Suzuki (1999b) (abbreviated hereinafter as Saito-Suzuki’s medium) (Table 1). The number of meristems per petri dish was 15; 9–20 petri dishes were prepared using Caboni’s callus induction medium; 6–19 petri dishes were prepared for Saito-Suzuki’s callus induction medium (Table 2). The meristems were cultured on Caboni’s and Saito-Suzuki’s media for 20 days at 25°C in the dark and for 60 days at 25°C in the dark, respectively. The culture procedure followed methods described by Caboni et al. (2000) and Saito and Suzuki (1999b). The callus formation rate was determined before transferring the calli to the subsequent medium (shoot induction medium or callus propagation liquid medium). The procedures from shoot multiplication medium to callus induction medium were designated No. 1 to No. 6 of the callus culture procedure (Tables 1 and 2).
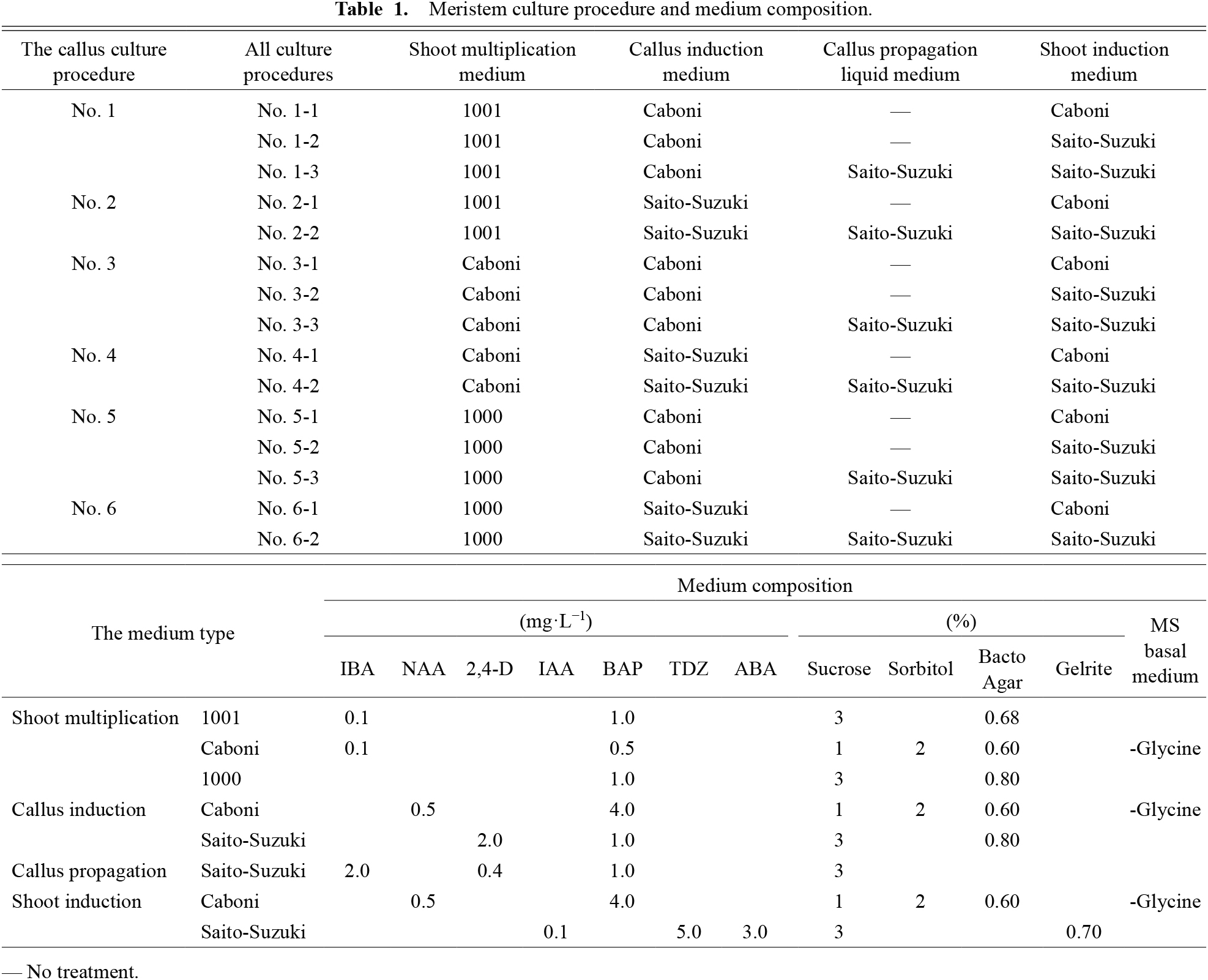
Meristem culture procedure and medium composition.
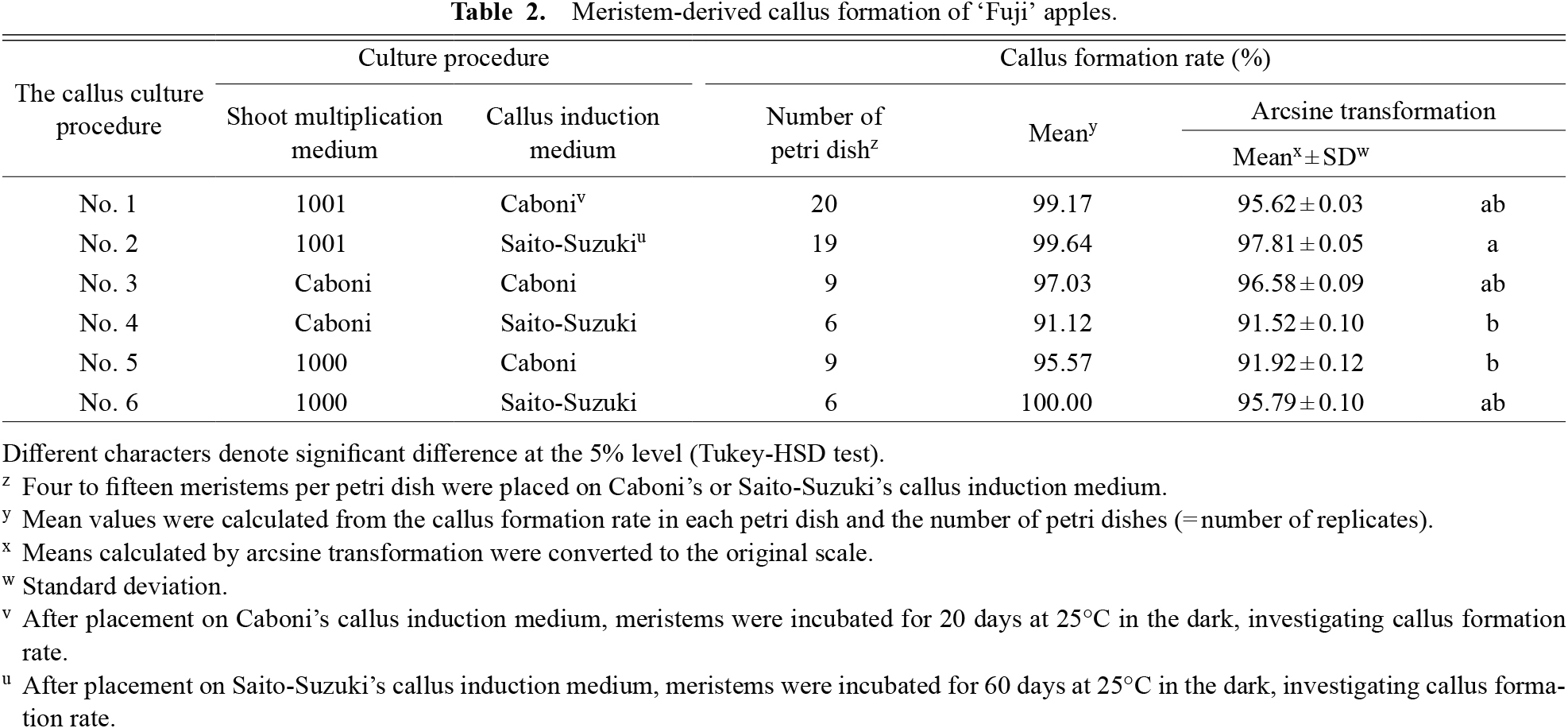
Meristem-derived callus formation of ‘Fuji’ apples.
Callus grown on Caboni’s or Saito-Suzuki’s callus induction medium were divided for culture using two procedures: with or without callus propagation liquid medium (Saito and Suzuki, 1999b). Calli transplanted in callus propagation liquid medium were shaken at 120 rpm for 12 h day length and were subcultured every 2 weeks for five times. Calli grown on Caboni’s or Saito-Suzuki’s callus induction medium, and Saito-Suzuki’s callus propagation liquid medium were transferred to Caboni’s or Saito-Suzuki’s shoot induction medium at 4–15 calli per petri dish (Tables 1 and 3). Calli were grown on shoot induction medium at 25°C with 16 h day length, and monthly subculture. Shoot regeneration rates were examined 3 months after transfer. Procedures from shoot multiplication medium to shoot induction medium were designated as No. 1-1 to No. 6-2 for all culture procedures (Tables 1 and 3). Effects of each culture procedure on shoot regeneration rates were analyzed.
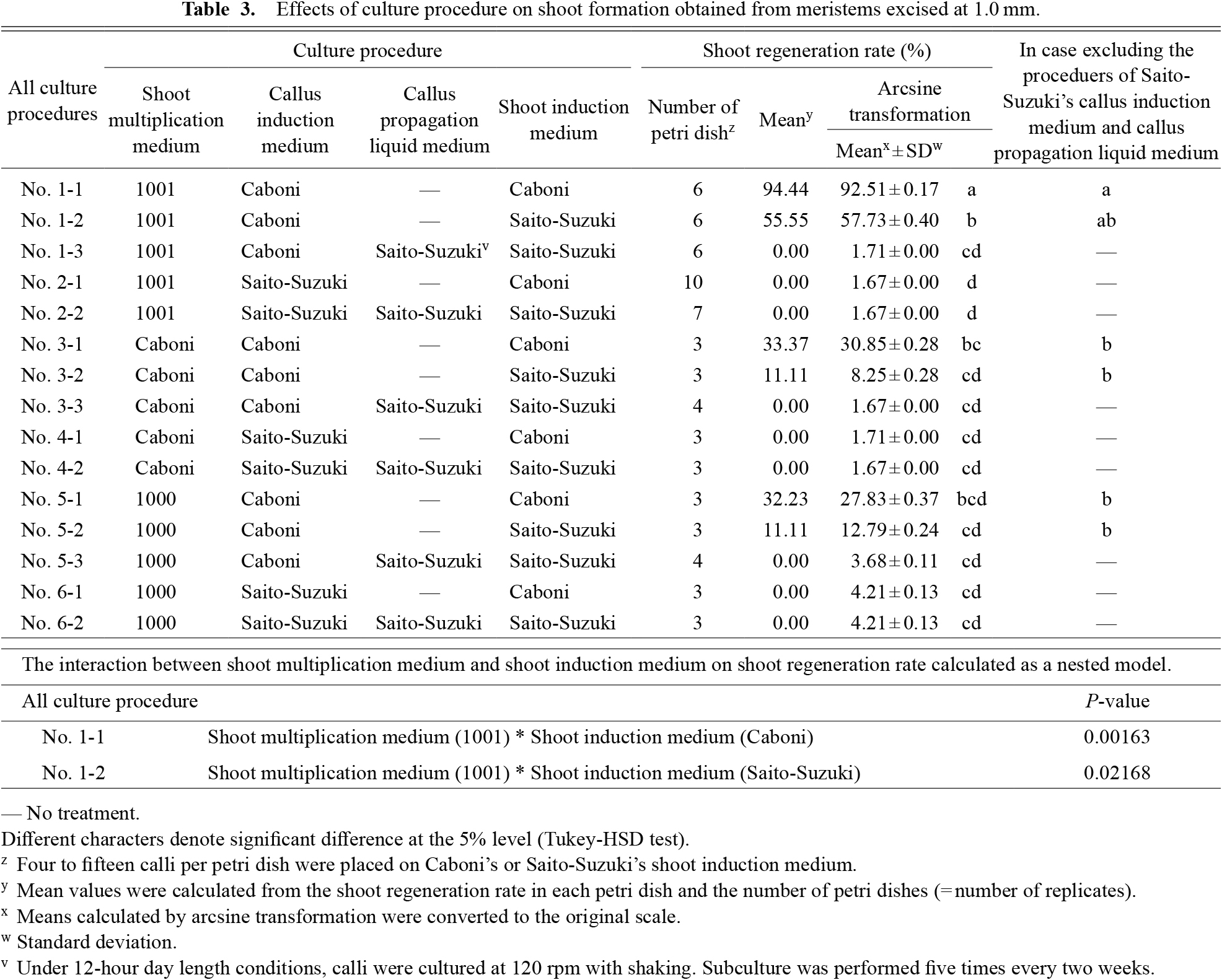
Effects of culture procedure on shoot formation obtained from meristems excised at 1.0 mm.
Experiments were conducted using ‘Fuji’ plantlets. The dates of primary culture are July 31, 2017, May 9, 2018, February 14, 2019, and June 4, 2019. Plantlets were maintained in subculture. The time from primary culture to meristem excision was from 2–34.5 months. Shoots maintained on 1001 shoot multiplication medium were transferred to dark conditions at 25°C for 1, 2, or 3 months before meristem excision. A control experiment was also prepared: without dark treatment on shoot multiplication medium. Meristems were obtained from whitened chloroplast-free shoots grown during the dark treatment (Fig. 1). Meristems were cut at 0.5 mm length, to exclude axillary buds. From the control without dark treatment in shoot propagation medium, meristems were excised at 0.5 mm long after 1 month of subculture. The excised meristems were placed on Carboni’s callus induction medium. Five meristems were placed per petri dish and incubated in the dark at 25°C for 20 days. Callus formation rates were examined before transplantation to shoot induction medium. Calli grown on Caboni’s callus induction medium were then transferred to Caboni’s or Saito-Suzuki’s shoot induction medium. Calli in shoot induction medium were incubated for 2 weeks under low light conditions (approx. 0.15 μmol·m−2·s−1 PPFD) at 25°C with a 16 h day length. Thereafter, plantlets were incubated under 16 h daylight with monthly subculture. Regeneration rates were investigated 3 months after transfer to the shoot induction medium. The effects of the period of dark treatment during shoot multiplication on the formation of callus and for shoots derived from the meristem of ‘Fuji’ were investigated. In addition, the effects of different shoot induction media on shoot regeneration were investigated for the callus.

Whitened shoots grown for 4 weeks under dark conditions.
Experiments were conducted using ‘Fuji’ plantlets. The primary culture date was April 13, 2020, and plantlets were maintained in subculture.
Shoots maintained on 1001 medium were transferred to 25°C dark conditions at the time of subculture (July 29, 2021), 2 months before cutting out meristems. The meristems were then cut from the shoots (October 8, 2021). The procedures from meristem excision to placement on shoot induction medium is as previously described in section 1. However, only Saito-Suzuki’s shoot induction medium was used. In the dark treatment, 2, 4, and 6 weeks of dark treatment were used for the shoot induction medium, then calli were incubated for 2 weeks under low light conditions of 16 h day length, followed by a 16 h light condition (approx. 45 μmol·m−2·s−1 PPFD). As a control, the calli were incubated immediately after transplanting at 25°C for 2 weeks under low light conditions with 16 h day length, followed by incubation under light conditions. In all treatments, subculture was performed each month. Three months after transplantation to shoot induction medium, the regeneration rates were investigated. Effects of the period of dark treatment during shoot induction on the formation of shoot from callus derived from the meristem of ‘Fuji’ were investigated.
3. Statistical analysisStatistical analysis software was used (JMP; SAS Institute Japan Ltd., Tokyo, Japan). Differences in callus formation rates derived from meristems (Table 2), shoot induction rates (Table 3), and effects of all culture procedures on shoot regeneration (Table 3) were assessed using a multiple comparison test of Tukey-Kramer HSD test. Interactions between shoot multiplication medium and shoot induction medium on shoot regeneration rate were analyzed as a nested model. Effects of dark treatment for callus formation and shoot regeneration during shoot multiplication (Table 4) and during shoot induction (Table 5) were also assessed using the multiple comparison test of Tukey–Kramer HSD test. Effects of the presence or absence of dark treatment and type of shoot induction medium for shoot regeneration during shoot multiplication (Table 4) and during shoot induction (Table 5) were assessed using Student’s t-tests.

Dark period effects on shoot multiplication medium for callus and shoot formation derived from meristems excised at 0.5 mm.
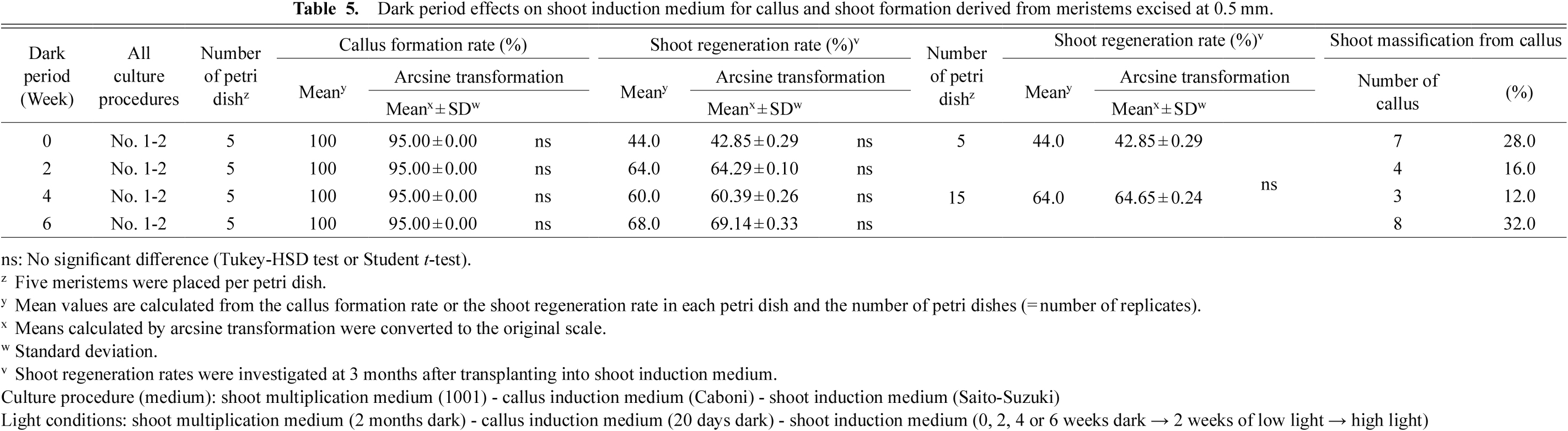
Dark period effects on shoot induction medium for callus and shoot formation derived from meristems excised at 0.5 mm.
Callus formation rates for the respective callus culture procedures are listed in Table 2. Callus formation rates were significantly higher for No. 2 than for No. 4, and No. 5. All six procedures showed high callus formation rates of more than 90%. The callus cultured on Saito-Suzuki’s callus induction medium did not regenerate shoots in subsequent procedures (Table 3). The callus was softer and more watery than callus cultured on Caboni’s callus induction medium (Figs. 2 and 3). Calli with high water content and a soft shape are unsuitable for shoot regeneration (Gabriele and Schwenkel, 2003). Calli cultured on Caboni’s callus induction medium that formed shoots in a subsequent procedure were hard calli. The long incubation period on Saito-Suzuki’s callus induction medium may be one factor limiting shoot regeneration.
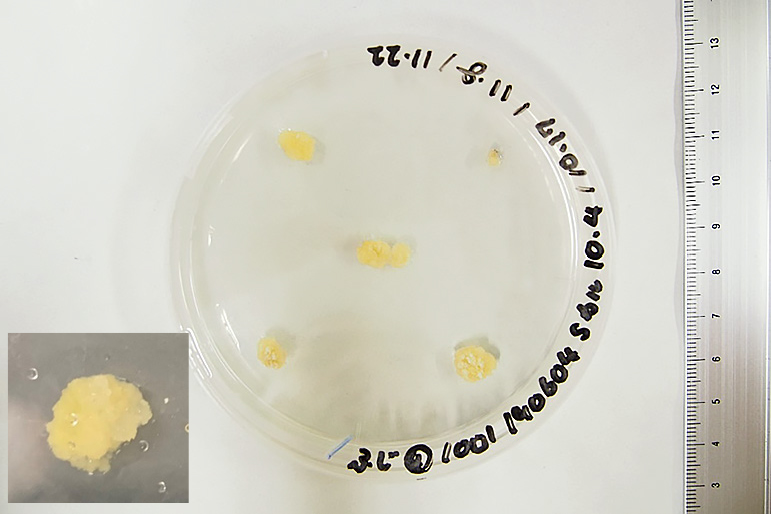
Callus formed with Saito-Suzuki’s callus induction medium.
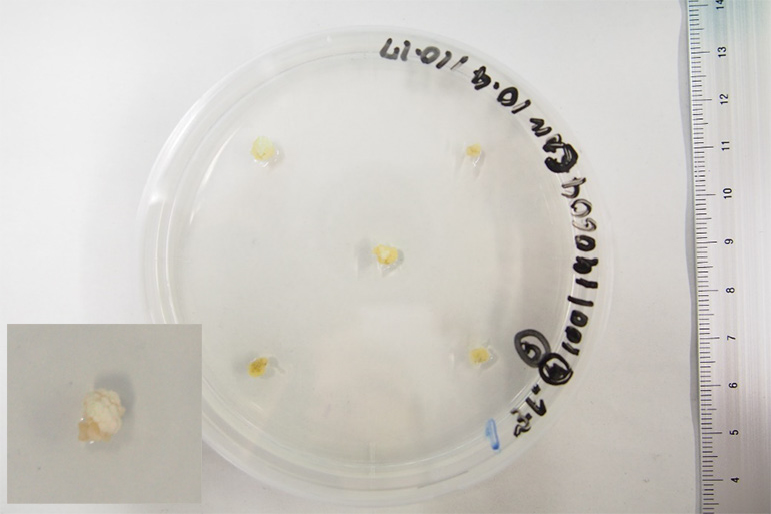
Callus formed with Caboni’s callus induction medium.
Shoot regeneration rates for all culture procedures No. 1-1 to No. 6-2 are listed in Table 3. The shoot regeneration rate was significantly higher in No. 1-1 than in the other treatments, followed in order by No. 1-2, No. 3-1, No. 5-1, No. 3-2, and No. 5-2. No shoot formation was observed using the other culture procedures.
(1) Reanalysis of the effects of the culture procedure excluding procedures using Saito-Suzuki’s callus induction medium and callus propagation liquid mediumWe reanalyzed the data to elucidate the effects of shoot multiplication medium and shoot induction medium on regeneration when callus propagation liquid medium and Saito-Suzuki’s callus induction medium were excluded, i.e., the effects of three shoot multiplication media (1001, Caboni, and 1000), and two shoot induction media (Caboni and Saito-Suzuki) (Table 3). Interactions between shoot multiplication medium and shoot induction medium on shoot regeneration rate were analyzed as a nested model.
Multiple comparison test results showed that the shoot regeneration rate of No. 1-1 was significantly higher than No. 3-1, No. 3-2, No. 5-1, or No. 5-2 (Table 3); No. 1-2 was next highest. No significant difference was found between No. 1-1 and No. 1-2. Media interactions were observed between the 1001 shoot multiplication medium and Caboni’s shoot induction medium (No. 1-1), and between the 1001 shoot multiplication medium and Saito-Suzuki’s shoot induction medium (No. 1-2). The results indicated the regeneration rates is higher in cases where the shoot multiplication medium and shoot induction medium are 1001 and Caboni’s (No. 1-1), and 1001 and Saito-Suzuki’s (No. 1-2) (Table 3) than in the other procedures.
(2) Selection of effective culture proceduresBased on these results, a suitable procedure for shoot regeneration from callus is the following: shoot multiplication on 1001 medium followed by Carboni’s callus induction medium, without callus propagation on liquid medium, and then Caboni’s shoot induction medium. As shoot regeneration rate was sufficiently high when using Saito-Suzuki’s shoot induction medium, it was also added to the investigation in the following experiments.
2. Effect of dark treatment on shoot regeneration of meristem-derived callus on shoot multiplication medium and shoot induction mediumBased on results presented in Table 3, experiments were conducted to investigate the effects of dark treatment during shoot multiplication and shoot regeneration using procedures No. 1-1 and No. 1-2, which demonstrated an effective regeneration response from meristem-derived callus.
1) Investigation of dark treatment period with shoot multiplication mediumEffects of the period of dark treatment with shoot multiplication medium on the formation of callus derived from the meristem of ‘Fuji’ are shown in Table 4. Callus formation rates, in all treatments, were higher than 92%. No significant difference was found. The procedure for dark treatment 0 month is the same procedure used for No. 1-1 and No. 1-2 in Table 3. However, the shoot regeneration rates were inferior to those in Table 3. For the experiment in Table 3, the shoot apexes were cut at 1.0 mm, which may include axillary buds, potentially increasing shoot formation rate. In this experiment (Table 4), the shoot apex sizes were 0.5 mm, thus only the meristems were excised, avoiding axillary bud inclusion.
The highest shoot regeneration rates for Caboni’s shoot induction medium (No. 1-1) and Saito-Suzuki’s (No. 1-2) were 20% after 2 months of dark treatment and 40% after 3 months of dark treatment, respectively (Table 4). For Saito-Suzuki’s shoot induction medium, a significant difference was identified in shoot regeneration rates between the normal light treatment and 1–3 months of dark treatment. In contrast, Caboni’s shoot induction medium yielded no significant difference in regeneration rates between dark treatment periods. The effects due to the presence or absence of dark treatment with the shoot multiplication medium on shoot regeneration were investigated (Table 4). In the procedures of 1001 shoot multiplication medium and Caboni’s callus induction medium followed by Caboni’s or Saito-Suzuki’s shoot induction medium, shoot regeneration rates were significantly higher with dark treatment for shoot multiplication medium (Table 4). Shoot regeneration rates were significantly higher for the procedure using Saito-Suzuki’s shoot induction medium than for the procedure with Carboni’s shoot induction medium (Table 4). From these results, it was inferred that a suitable procedure for regeneration from the meristem-derived callus consists of excising the meristems of shoots grown in the dark on shoot multiplication medium for several months, followed by culture on Carboni’s callus induction medium, and then on Saito-Suzuki’s shoot induction medium.
As described above in section 1, it was inferred that the procedure using Carboni’s callus induction medium and Carboni’s shoot induction medium was best for shoot regeneration (Table 3). However, the procedure using Carboni’s callus induction medium and Saito-Suzuki’s shoot induction medium was inferred as optimal for shoot regeneration when the dark treatment was used for the shoot multiplication medium. These results suggest that the appropriate medium may differ depending on the presence or absence of dark treatment. In the procedure followed using Carboni’s callus induction medium and Saito-Suzuki’s shoot induction medium, shoots regenerated even 6 months after callus formation (data not shown). Thus, the meristem-derived callus maintained regeneration ability for a long period.
2) Investigation of dark treatment period in shoot induction mediumThe following procedure was used for this experiment. Shoot-meristems were excised after 2 months of dark treatment in shoot multiplication medium with subculture on Carboni’s callus induction medium and Saito-Suzuki’s shoot induction medium.
The effects of the period of dark treatment with the shoot induction medium on shoot regeneration of meristem-derived callus from the ‘Fuji’ cultivar are listed in Table 5. Callus formation rates were 100% in each treatment. No significant difference was found in shoot regeneration rates among dark treatment periods. However, the highest shoot regeneration rate of 68.0% was observed for the 6-week dark treatment on shoot induction medium. Based on earlier reports, this is a high shoot regeneration rate for callus obtained from cultivated apple cultivars (Liu et al., 1994; Saito and Suzuki, 1999b). The shoot regeneration rates for the dark treatment of shoot induction medium (Table 5) were higher than any shoot regeneration rates obtained without dark treatment of shoot induction medium, listed in Table 4.
The effect of dark treatment for shoot induction medium on shoot regeneration was investigated (Table 5), but no significant difference was observed. However, the average regeneration rate was about 20% higher for the dark treatment than for the non-dark treatment. The shoot regeneration rate was 44% when the period of dark treatment with the shoot induction medium was 0 weeks. This is almost equal to the 36% shoot regeneration rate (Table 4) for the 2 months dark treatment for the shoot multiplication medium.
3. Relation between regeneration potential and plant material, and prospects 1) Findings from earlier studiesFew successful cases of shoot regeneration from apple callus are reported (Caboni et al., 2000; Liu et al., 1994; Rugini and Muganu, 1998; Saito and Suzuki, 1999a, b). In one of these studies, Liu et al. (1994) used callus derived from ‘Fuji’ cotyledons. Although the cotyledons have high regeneration potential, this experiment was not considered in the present study as the genetic composition of cotyledon-derived individuals differs from the seed parent cultivar. Rugini and Muganu (1998) achieved high shoot regeneration rates using callus derived from ‘Golden Delicious’, maintained for 3 years by subculture. A special procedure employing a stereomicroscope to induce callus by cutting petioles from adventitious shoots regenerated by leaf segment culture. The callus retained shoot regeneration ability for about 1 year. If this process was applicable to many cultivars and were simplified, it would be an excellent experimental system. The procedures of experiments reported by Saito and Suzuki (1999b) and Caboni et al. (2000), referred to for the present experiments, both use shoot regeneration systems from meristem-derived callus. The experiment procedures described by Caboni et al. (2000) constitute a simple and excellent method. Changing the gelling agent of the shoot induction medium to Gelrite is expected to improve shoot regeneration rate. Also, cefotaxime (CX), added to the callus induction medium by Caboni et al. (2000), was not added for our experiment as it markedly inhibits shoot regeneration (Komori et al., 2009). The system used by Saito and Suzuki (1999b) for experimentation includes many procedures identical to those for protoplast-derived shoot regeneration experiments (Saito and Suzuki, 1999a). The callus propagation liquid medium that was ineffective in our study is presumed to select for callus with high shoot-formation ability by protoplast culture. The callus propagation liquid medium was regarded as unnecessary in the protocol for shoot regeneration experiments using callus without protoplasts. Saito and Suzuki (1999b) did not perform the shoot propagation procedure, instead inducing callus directly by meristem excision from shoots grown in a greenhouse. This manipulation may result in increased shoot-forming ability of the callus. As sorbitol is effective in apple shoot regeneration (Li et al., 2011), the addition of sorbitol to Saito-Suzuki’s shoot induction medium is expected to improve shoot regeneration rates. The high shoot regeneration rate (68.0%) achieved with our experiment procedure is attributed to the use of shoot multiplication medium 1001, the effectiveness of Caboni’s callus induction medium, Saito-Suzuki’s shoot induction medium, and dark treatment on shoot multiplication stage and shoot induction stage. Presumably, dark treatment suppressed organ differentiation at the growth point, facilitating the induction of dedifferentiated callus with totipotency (Iwase et al., 2016).
2) Relation between plant materials and regeneration abilityIn most cases, leaf segments are used as materials for shoot regeneration experiments in apple tissue culture. In transformation experiments using Agrobacterium method, leaf segments are also infected with Agrobacterium (Hammerschlag et al., 1997; Komori et al., 2009; Li et al., 2011; Maximova et al., 1998; Norelli et al., 1996; Yao et al., 1995). For genome editing, the shoot regeneration system with protoplasts, a one-cell derived regeneration system that avoids chimerism, is an effective culture system. Microinjection and electroporation are applicable to this culture system (Malony et al., 2016; Osakabe, 2018). In apples, shoot regeneration from protoplasts is more difficult than shoot regeneration from callus, with very few successful cases reported (Saito and Suzuki, 1999a). The plants used in shoot regeneration experiments using protoplasts and callus in Saito and Suzuki (1999a, b) were derived from the meristems of Malus prunofolia Borkh. Marbakaido and the apple cultivar ‘Fuji’ (M. × domestica Borkh.). In evaluating shoot regeneration from callus, the regeneration rate of Marbakaido was superior to that of ‘Fuji’. Both M. prunofolia Borkh. and M. × domestica Borkh. are classified among Eumalus Zabel (Rehder, 1986). Marbakaido, which has rooting ability from cuttings, is used as a rootstock for apples. Rooting ability from cuttings is often used as an index of juvenility in fruit trees. Branches taken from unflowered seedlings within a few years of germination are known to root easily, even in cultivated apples (Kuroda, 2002; Westwood, 1978). However, rooting from cuttings of apple cultivars after reaching the adult phase is difficult. ‘Fuji’ used for the present study for shoot regeneration from meristem-derived callus was maintained in vitro by subculture. The meristem is a tissue with high regeneration potential, similar to nucellus cells. Few reports describe shoot regeneration from apple nucellus cell derived callus (Saito et al., 1989). Immature seeds are used to induce callus from the nucellus cells (Kawato et al., 2022; Saito et al., 1989). It is speculated that the high regeneration potential of the nucellus cells is associated with juvenility. The embryogenic callus or adventitious embryo of carrot is derived from the hypocotyls, cotyledons, and meristems of the seedling about a week after sowing (Kaimori and Ishihara, 1991; Kamada et al., 1993). While the callus used in the citrus shuttle callus system (Hidaka and Omura, 1989) is an embryoid derived from the immature embryo and pollen. Grapevine embryogenic callus is derived from the filaments of unflowered buds and from unfertilized ovules (Nakajima et al., 2000, 2020). All are young tissues with high regeneration potential. In addition, grapes are fruit trees with rooting ability by cutting. These findings demonstrate the desirability of using young tissues with high regeneration potential for shoot formation from callus or protoplasts. For plants described in the above section 1, primary culture was conducted on May 9, 2018. The meristems were excised 7.5 months later. In Section 2. 2), meristems were excised 18 months after the primary culture. The short period between primary culture to meristem excision in these two experiments may be one reason for the higher rate of shoot regeneration than in conventional shoot regeneration from callus experiments. In Section 2.1, the primary cultures were conducted on July 31, 2017, May 9, 2018, February 14, 2019, and June 4, 2019. The time from primary culture to meristem excision was from 2 months to 34.5 months. Although this experiment did not investigate differences in shoot regeneration rates at different times of primary culture, the dark treatment likely enhanced shoot regeneration ability. Thus, to develop practical experimental systems for regeneration from callus and protoplasts, selection of plant material with high regeneration potential, improvement of the culture medium, and application of dark treatment are each important.
3) Shoot massification from callusThe procedure developed in this experiment is expected to be used for genome editing in direct transfection methods such as a particle bombardment. However, most of the meristem-derived calli only differentiate 2–3 shoots per callus. In this experiment, a few shoot-massified calli were observed on both Caboni’s and Saito-Suzuki’s shoot induction media (Table 5; Fig. 4). Identifying optimal culture conditions for shoot massification from callus remains to be achieved. In this experiment, callus shoot regeneration ability was maintained for at least 6 months (data not shown). To establish a production system for shoot regeneration from callus, it is necessary to establish a culture system that maintains callus and ensures shoot regeneration potential. This study may be useful not only for ‘Fuji’ but also for achieving shoot regeneration from callus derived from many apple cultivars.

Shoot massification from callus derived from meristem. Culture procedure: Cultured for 1 month under dark conditions in shoot multiplication medium → Caboni callus induction medium → Saito-Suzuki shoot induction medium
The authors are grateful to members of the horticultural laboratory of Faculty of Agriculture, Iwate University for their co-operation.