2023 年 92 巻 3 号 p. 290-298
2023 年 92 巻 3 号 p. 290-298
Purple sweet potatoes are rich in the purple pigment anthocyanin. In recent years, it has been reported that the anthocyanin content of the same variety varies depending on the place of production. Therefore, to investigate the effect of soil temperature on the anthocyanin content of sweet potato tuberous roots, two types of covering materials, black and white mulch films, were used in the same field. The vines were planted in May, June and July; the cultivation period was set to 120–180 days and tuberous roots were harvested in September, October and November. The average soil temperature ranged from 22.9 to 26.5°C, with the white mulch having lower soil temperatures than the black mulch. The tuberous root yield increased with longer cultivation periods; the tuberous root yield in the May–November plot with a 180-day cultivation period was about twice that of the June–October and July–November plots with a 120-day cultivation period. The anthocyanin content of the tuberous root was negatively correlated with the average soil temperature; the test plots harvested in November had a higher anthocyanin content than the other test plots. In particular, the anthocyanin content of sweet potato cultivated in white mulch in July–November was about twice that cultivated in May–September. Although the factors that increase the tuberous root yield (prolonged cultivation period) are different from those that increase the anthocyanin content (lower temperature range), the tuberous root yield showed a larger effect on the total anthocyanin yield. Since the cultivation period needs to be prolonged to increase tuberous root yield, it would consequently increase the anthocyanin yield. The quality of the harvest was better under lower soil temperature as it led to an increase in the anthocyanin yield. Therefore, it was suggested that anthocyanin yields could be maximized by extending the growing season and harvesting at lower soil temperatures.
Several local varieties of purple sweet potato such as ‘Tanegashima-murasaki’ and ‘Bise’ with anthocyanin content not too high for table use have been cultivated (Oki et al., 2002). For use as pigment raw material, a variety ‘Ayamurasaki’ with a high anthocyanin content is cultivated. Products such as beverages and sweets using the purple sweet potato pigment have been developed, leading to the development of newer improved varieties in terms of pigmentation and other traits. ‘Murasakimasari’ is a variety derived from a cross between ‘Ayamurasaki’ and a high starch variety ‘Shiroyutaka’. It has the same pigment content as ‘Ayamurasaki’ and is also characterized by a high starch content (Kumagai et al., 2002). In addition, ‘Murasakimasari’ is a high-yielding variety and is therefore used as a raw material for shochu; it is the most commonly cultivated variety of purple-flesh-colored sweet potato in Japan (MAFF, 2021).
The major anthocyanin of purple sweet potato is a pigment called YGM (YGM-1–6), which was discovered in ‘Yamagawamurasaki’ and constitutes a glycoside of cyanidin (Cy) or peonidin (Pn). YGM-1–6 is also a major anthocyanin in ‘Murasakimasari’ (Oki et al., 2017; Terahara et al., 1999; Yoshinaga et al., 1999) (Fig. 1). Anthocyanins are usually not very stable, but purple sweet potato anthocyanins are highly stable because they are glycosides with sophoroside at the C-3 position and glucoside at the C-5 position of aglycones, along with the presence of organic acids (Cevallos-Casals and Cisneros-Zevallos, 2004; Qian et al., 2017). Purple sweet potato anthocyanins have various health benefits, such as antioxidant, antimutagenic, anti-inflammatory and antidiabetic activity, as well as hepatoprotective effects (Jiang et al., 2020; Oki et al., 2002; Suda et al., 2003; Wang et al., 2017; Yoshimoto et al., 2001).

Structure and molecular weight of anthocyanins (YGM-1–6) in the ‘Murasakimasari’ variety of sweet potato. Cy: Cyanidin, Pn: Peonidin, CH3: Methyl group, FA: Ferulic acid, CA: Caffeic acid, PHB: p-Hydrocybenzoic acid.
Because health functionalities add value to processed products, processed purple sweet potato products with a high anthocyanin content are preferred. In particular, the use of purple sweet potato varieties as pigmented raw materials requires that they have a stable and high anthocyanin content, which depends on the pigment production capacity of the varieties. Furthermore, higher tuberous root yields are preferred for varieties with similar pigment content, so improving tuberous root yield is always an important consideration. In recent years, some studies have reported that the anthocyanin content of the same purple sweet potato varieties varies depending on the cultivation area in Japan (Ishiguro et al., 2022; Oki et al., 2002). In addition, it is known that the color value, which is a guideline value for breeding sweet potatoes based on anthocyanin content, is highly temperature sensitive (Kobayashi et al., 1998). However, the relationship between the tuberous root yield of purple sweet potato and the anthocyanin content has not yet been established. Therefore, we designed a field experiment to understand the influence of soil temperature on tuberous root yield and anthocyanin content of sweet potato.
The experiment was conducted for three years between 2017 and 2019 at the Miyakonojo research station, Kyushu Okinawa Agricultural Research Center, NARO (Miyakonojo, Miyazaki, Japan; longitude, 131°01'E; latitude, 31°75'N). The sweet potato variety ‘Murasakimasari’ was used. Seed tubers were planted in a greenhouse in March (6 pieces × 150 cm placed at 20 cm intervals), and the shoots were grown until May. Stem cuttings with seven nodes were collected on the planting day in May and planted to ridges width of 75 cm with 35 cm spacing between plants. Black mulch (Okura Industrial Co., Ltd., Kagawa, Japan, width 95 mm × length 200 m, thickness 0.03 mm; used for all three years) and white mulch (Okura Industrial Co., Ltd., width 95 mm × length 200 m, thickness 0.025 mm; used between 2018 and 2019 only) plots were created. A nitrogen:phosphoric acid:potassium ratio of 4.8:7.2:12.0 g·m−2 was applied with the basal fertilizer (cattle manure compost, 1 kg·m−2). The vines (leaves and stem) were planted in the fields in early May, June (Jun) and July (Jul), and harvested in early September (Sep), October (Oct) and November (Nov). The cultivation period was 120 days or more, which is a common period for sweet potato cultivation. By changing the month of planting and harvesting in the same field, test plots with different average soil temperatures were set up. During the cultivation period, the soil temperature 10 cm below the top of the ridge was measured every hour using a temperature logger SK-L200TH (Sato Keiryoki Mfg. Co., Ltd., Tokyo, Japan). Each plot was designed with 5 ridges × 6 plants. We harvested a total of 12 plants (3 ridges × 4 plants) from the center of each plot to measure the foliage weight and tuberous root yield. Tubers weighing less than 50 g, which are of no commercial value, were excluded from tuberous root yield measurements. After post-harvest storage at 15°C for one week, samples were washed, freeze-dried in LFD-1200DNS1 (Laytant life science Co., Ltd., Tokyo, Japan) and crushed using a mixer and stored at −20°C until they were used for anthocyanin analysis. The moisture content was also obtained by freeze-drying.
Growth rate (GR) was determined by dividing the tuberous root yield (kg/10 a) by the number of growing days and expressed in kg/10 a·day−1.
Anthocyanin extraction and quantitative analysisOne hundred milligrams of freeze-dried powder (FDP) from purple sweet potato were added to 5 mL of 40% methanol solution containing 0.5% trifluoroacetic acid (v/v). This was thoroughly mixed using a vortex mixer and sonicated at 37°C for 5 min. The samples were then incubated at 37°C for 10 min. After centrifugation at 2,000 × g for 10 min, the supernatant was collected and used to measure the anthocyanin content.
Anthocyanins were measured using high-performance liquid chromatography (HPLC) as described by Oki et al. (2017). Briefly, the extract was filtered through a polytetrafluoroethylene membrane (0.20 μm; Advantec, Tokyo, Japan); 10 μL filtrate was injected into the HPLC system and eluted as described below. The HPLC system consisted of a DUG-14A degasser, SIL-10A XL auto-injector, CTO-10A column oven, SPD-M10AVP diode array detector, CBM-20A communications bus module and two LC-10AT pumps (Shimadzu Co., Kyoto, Japan). The system was controlled using a Lab solution (version 5.73) workstation (Shimadzu Co.). A Cadenza CD-C18 (250 mm × 4.6 mm i.d., 3 μm particles; Imtakt, Kyoto, Japan) column was used; the temperature of the column oven was set to 35°C. The anthocyanins were detected by absorbance at 520 nm. The mobile phase consisted of water containing 0.6% (v/v) formic acid (A) and 50% (v/v) acetonitrile containing 0.6% (v/v) formic acid (B). Elution was performed with a linear gradient of (B) as follows: 20% to 50% from 0 to 50 min, with a flow rate of 0.6 mL·min−1 for (B). The concentrations of the eluted anthocyanins were determined as YGM-6 equivalents (equiv.) using YGM-6 as the standard substance.
Based on the moisture content of each sample, the anthocyanin content was converted by calculation from per dry weight (Freeze dried powder, FDP) to per fresh weight (FW). The anthocyanin yield was calculated using the following formula. Anthocyanin yield (kg YGM-6 equiv./10 a) = tuberous root yield (kg/10 a) × anthocyanin contents (mg YGM-6 equiv.·kg−1 FW) × 10−6.
Statistical analysisFor the experimental results, one-way analysis of variance (ANOVA) was performed, after which a multiple comparison test was performed using the Tukey-Kramer method as a significant difference test between treatment plots. Two-way ANOVA was performed for tuberous root yield, GR and anthocyanin content versus cultivation period and mulch color. Regression lines and correlation coefficients r were obtained for soil temperature and total anthocyanin content or each anthocyanin component content (YGM-1–6) from scatter plots. Correlation analysis was judged by the level of significance of the Pearson’s correlation coefficient r. The level of significance was set to 5% or less (P ≤ 0.05).
Table 1 shows the soil temperature for three years (two years for white mulch) by cultivation period when the planting time was set to May, Jun and Jul and the harvest time was set to Sep, Oct and Nov. The average soil temperature in each plot varied from 23–26.5°C for black mulch and 22.9–25.6°C for white mulch. During the same cultivation period, the average soil temperature of white mulch was lower than that of black mulch. In addition, the average soil temperature of the test plots for which harvest time was set to November was lower by one degree or more compared to that of other treatment plots.

Variations in average soil temperature by cultivation period and agricultural mulch film color.
As shown in Table 2, significant differences in tuberous root yield and cultivation period were obtained in 2018 and 2019, but only in 2019 were significant differences observed in tuberous root yield and mulch color. The highest tuberous root yield was 4373.4 kg/10 a on average during 180-days of cultivation from May–Nov in black mulch. The yields of the 150-day cultivation period from May–Oct and Jun–Nov were more than 3000 kg/10 a. The lowest tuberous root yield was 1800–2600 kg/10 a during the 120-day cultivation period from May–Sep, Jun–Oct and Jul–Nov. Tuberous root yields were significantly higher for white mulch than black mulch in the Jun–Oct test plots in 2018 and 2019. Significant annual differences were observed in the test plots from May–Nov, and tuberous root yields were highest in both mulch types. The tuberous root yields for each cultivation period were the same. The average GR of tuberous roots exceeded 19 kg/10 a·day−1 except from Jun–Oct and Jul–Nov (Table 3). GR also behaved similarly to the tuberous root yield, indicating that long-term cultivation promotes root enlargement. GR was also significantly higher for white mulch than black mulch in the Jun–Oct cultivation test plot. In addition, the relationship between GR and planting or harvesting month was examined and it was found that planting in May had significantly higher GR values regardless of mulch color, while no significant correlation was obtained for harvesting month (Table 4). From these results, it was clear that the tuberous root yield of ‘Murasakimasari’ more than doubled when the cultivation period was extended from 120 to 180 days.
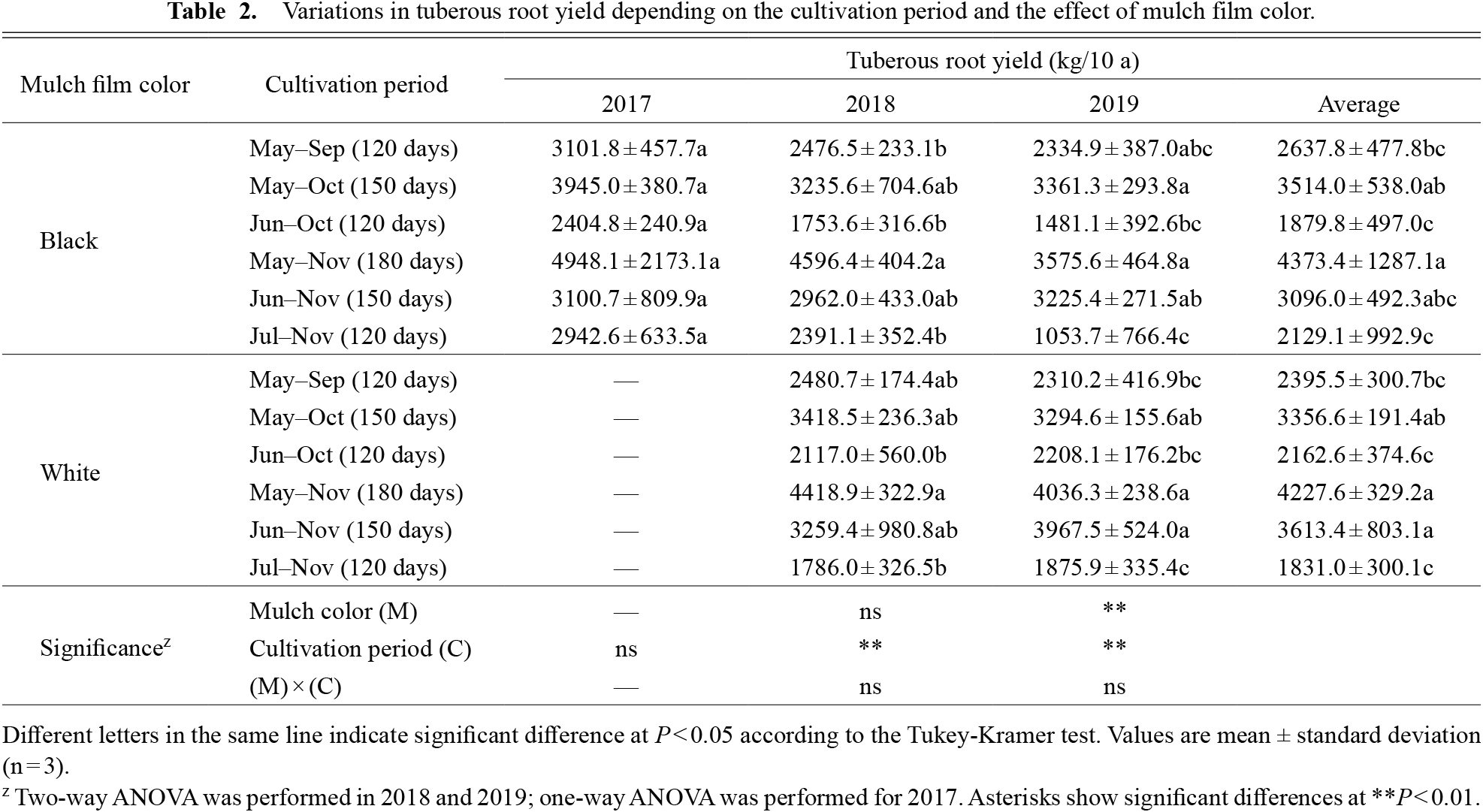
Variations in tuberous root yield depending on the cultivation period and the effect of mulch film color.
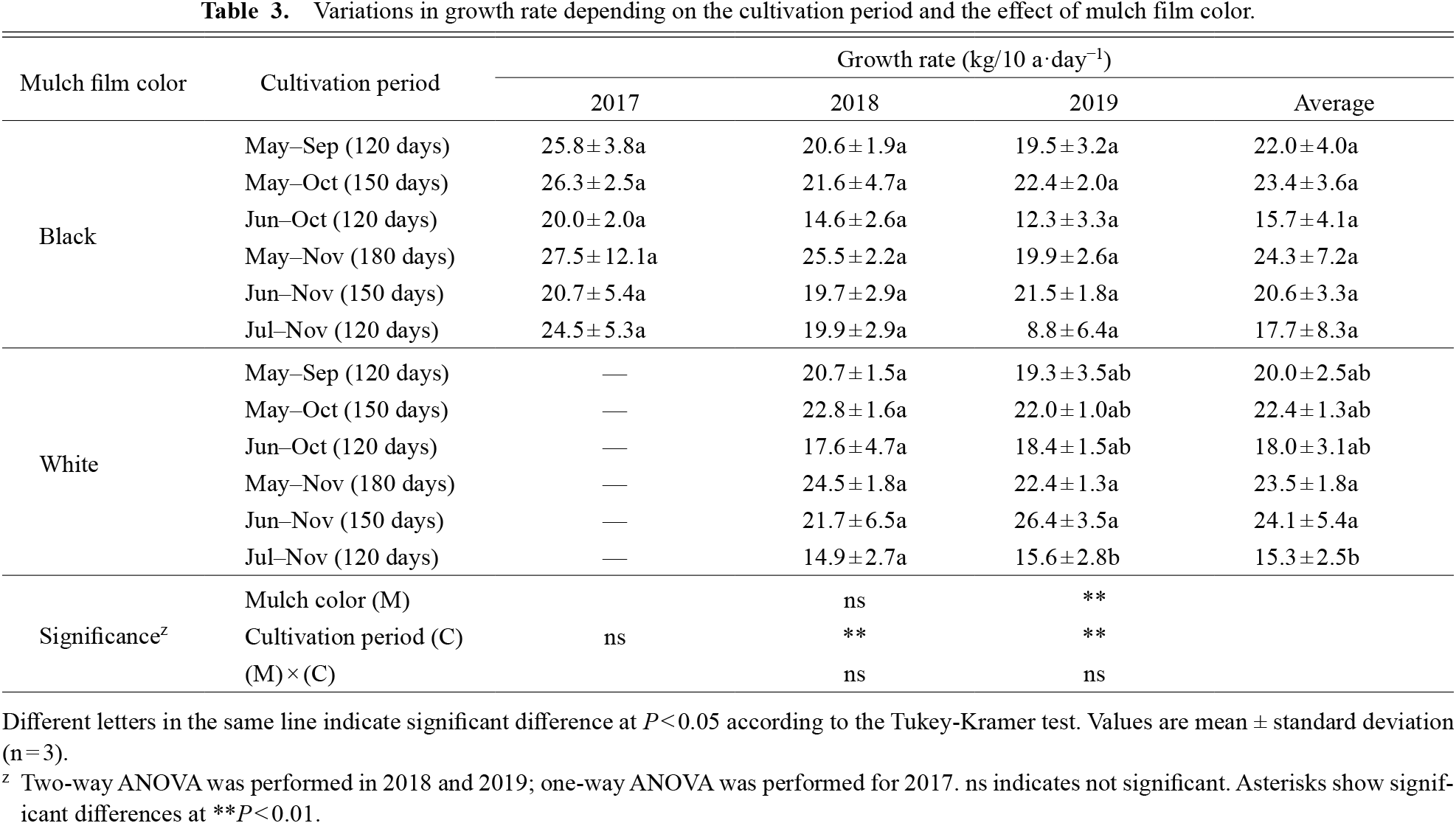
Variations in growth rate depending on the cultivation period and the effect of mulch film color.
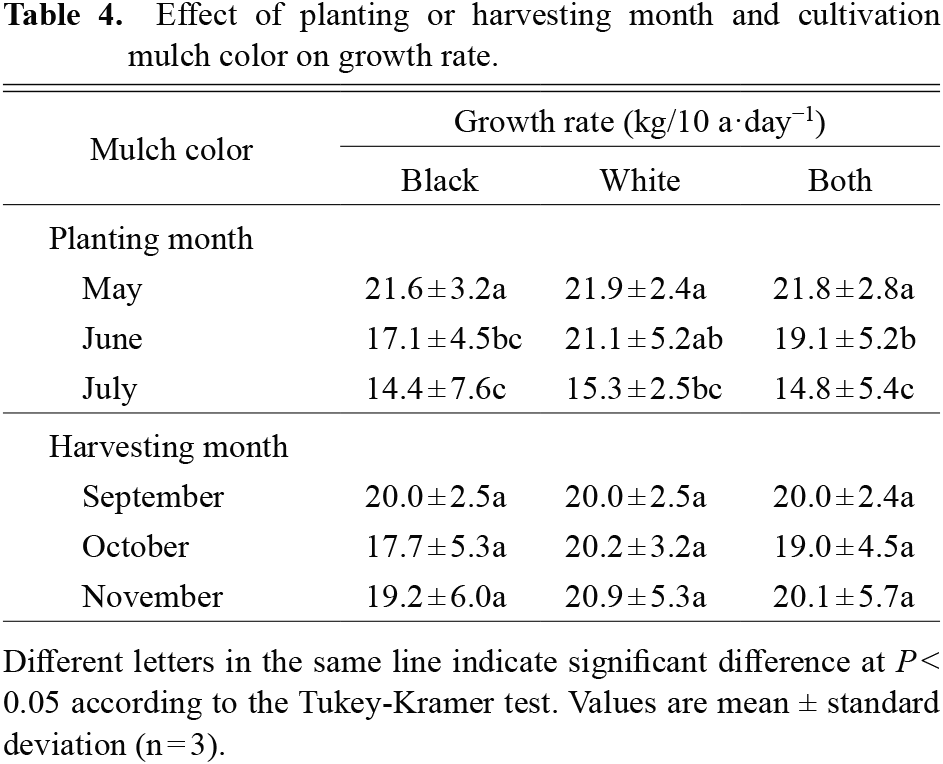
Effect of planting or harvesting month and cultivation mulch color on growth rate.
The anthocyanin content also changed depending on the cultivation period (Table 5). The highest anthocyanin content of 18.3 mg YGM-6 equiv.·g−1FDP was obtained for sweet potato cultivation in white mulch from Jul–Nov (120 days), which was close to that of the 15.8 mg YGM-6 equiv.·g−1FDP obtained from Jun–Nov (150 days). The lowest anthocyanin content of 8.9 mg YGM-6 equiv.·g−1FDP was obtained from the cultivation in May–Sep with white mulch. The maximum and minimum anthocyanin content of the annual average showed a marked change of 1.5 times in black mulch and 2 times in white mulch, respectively. The accumulation of anthocyanins could not be attributed to the length of the cultivation period. Rather, the November harvest plots tended to have higher anthocyanin content than the other test plots. Cultivations with different mulch colors for anthocyanin accumulation showed significant differences in both years, but only in 2018 also showed an interaction. This could be attributed to the different responses in terms of anthocyanin content in the Jul–Nov test plot to white or black mulch cultivation.
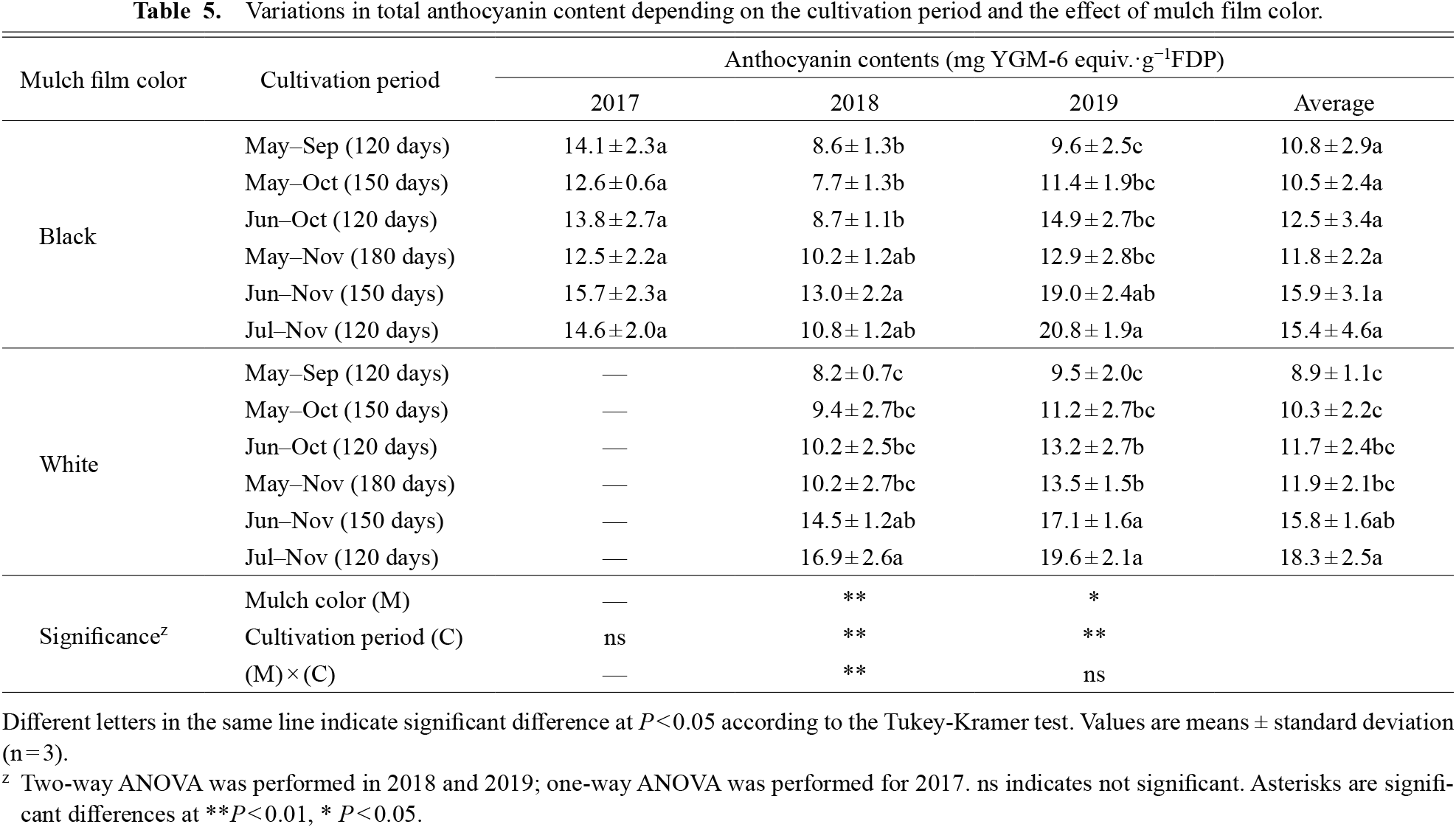
Variations in total anthocyanin content depending on the cultivation period and the effect of mulch film color.
As shown in Figures 2A and B, the anthocyanins were highest in YGM-5b in all cultivation periods, followed by YGM-6. The next highest content was that of YGM-5a and 4b, and the combined content of these four anthocyanins was ≥ 80% (Fig. 2C, D). As for aglycones, YGM-1–3 was attributed to Cy and YGM-4–6 with Pn (Fig. 1); therefore, the composition of ‘Murasakimasari’ was always ≥ 80% of Pn. The anthocyanin content varied with cultivation period and increased in the order of Sep < Oct < Nov harvesting, except for YGM-2 (black mulch) and YGM-5b (Fig. 2A, B). In the test plots harvested in November, the amount of Cy type YGM-1–3 showed an increase in the order of May ≤ June < July. Since the Cy/Pn ratio (%) of anthocyanin fluctuated by only a few percent due to soil temperature, the flesh color remained almost unchanged (Fig. 2C, D). YGM-5b composition (%) was decreased in the November harvest plots under both black and white mulch cultivation. Conversely, the compositions of YGM-1a, -1b, -3, and -4b increased in the November harvest plots.

Effects of mulch-film color and cultivation period on anthocyanin content and composition. A, The anthocyanin contents in black mulch cultivation (mg YGM-6 equiv.·g−1FDP). B, The anthocyanin contents in white mulch cultivation (mg YGM-6 equiv.·g−1FDP). C, Content ratio (%) of YGM-1–6 by black mulch cultivation period. D, Content ratio (%) of YGM-1–6 by white mulch cultivation period. The data show a three-year average for black mulch and a two-year average for white mulch cultivation. Different letters in the same line indicate significant difference at P < 0.05 according to Tukey-Kramer test.
Since the anthocyanin yield was calculated from the tuberous root yield and the anthocyanin content, there was a large difference between years (Table 6). However, the trend of anthocyanin yield under each cultivation period was very stable. The average anthocyanin yield over the three years in black mulch cultivation was high in May–Nov (20.8 ± 7.9 kg YGM-6 equiv./10 a), but low in Jun–Oct (8.6 ± 3.5) and Jul–Nov (9.8 ± 4.5). Similarly, the average anthocyanin yields in white mulch in May–Nov (19.9 ± 2.7) and Jun–Nov (20.9 ± 6.0) were high, whereas those in May–Sep (7.8 ± 1.2), Jun–Oct (9.4 ± 2.2) and Jul–Nov (11.0 ± 2.7) were low; these were 120-day cultivation subplots. It could be inferred that the anthocyanin yield was high in May–Nov and low in Jun–Oct and Jul–Nov. The differences between the highest and lowest anthocyanin yields were 2.4-fold (black mulch) and 2.7-fold (white mulch).
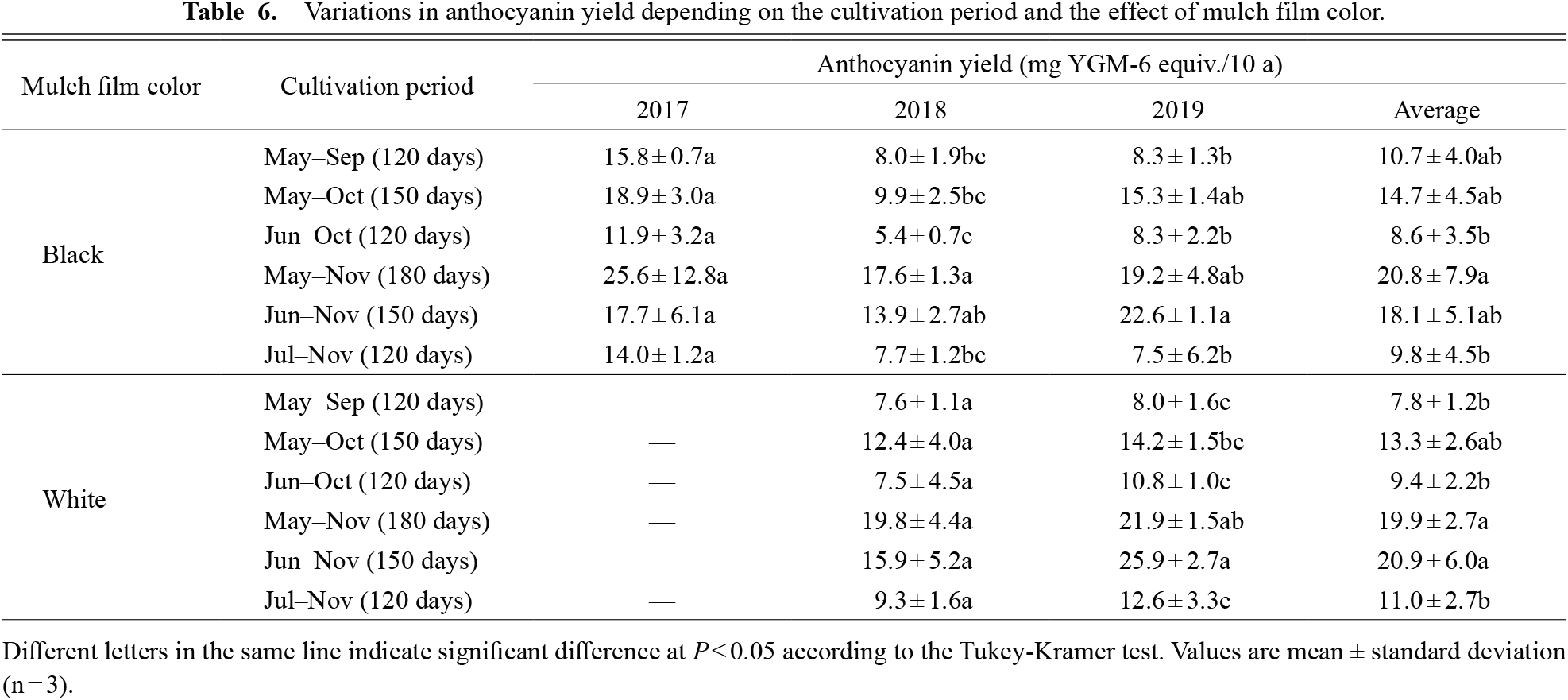
Variations in anthocyanin yield depending on the cultivation period and the effect of mulch film color.
The correlation between the total anthocyanin content and the average soil temperature during the cultivation period was investigated (Fig. 3). No significant correlation was obtained between the anthocyanin content and the soil temperature under black mulch cultivation, but a negative correlation of r = −0.784 (P < 0.01) was observed in the white mulch cultivation.

Relationship between average soil temperature and total anthocyanin content during the cultivation period. The equation and correlation coefficient are for white mulch and black mulch. Asterisks (**) indicate significance at P < 0.01.
Furthermore, the relationship between each composition of anthocyanins and the soil temperature was investigated (Fig. 4). There were significant negative correlations between YGM-1a, -1b, -3, -4b, -5a, -6 and the average soil temperature in both white and black mulch cultivation (P < 0.001, except for P < 0.05 for YGM-5a and -6 black mulch). No correlation was found between YGM-5b and the average soil temperature. Furthermore, there was no correlation between YGM-2 and the average soil temperature in black mulch cultivation, but a correlation (P < 0.05) in white mulch cultivation was observed. White mulch showed less variability in anthocyanin content at low temperatures than black mulch, which was reflected in the significant difference in the correlation between YGM-2, -5a, -6 and total anthocyanin content with respect to soil temperature (Figs. 3 and 4).
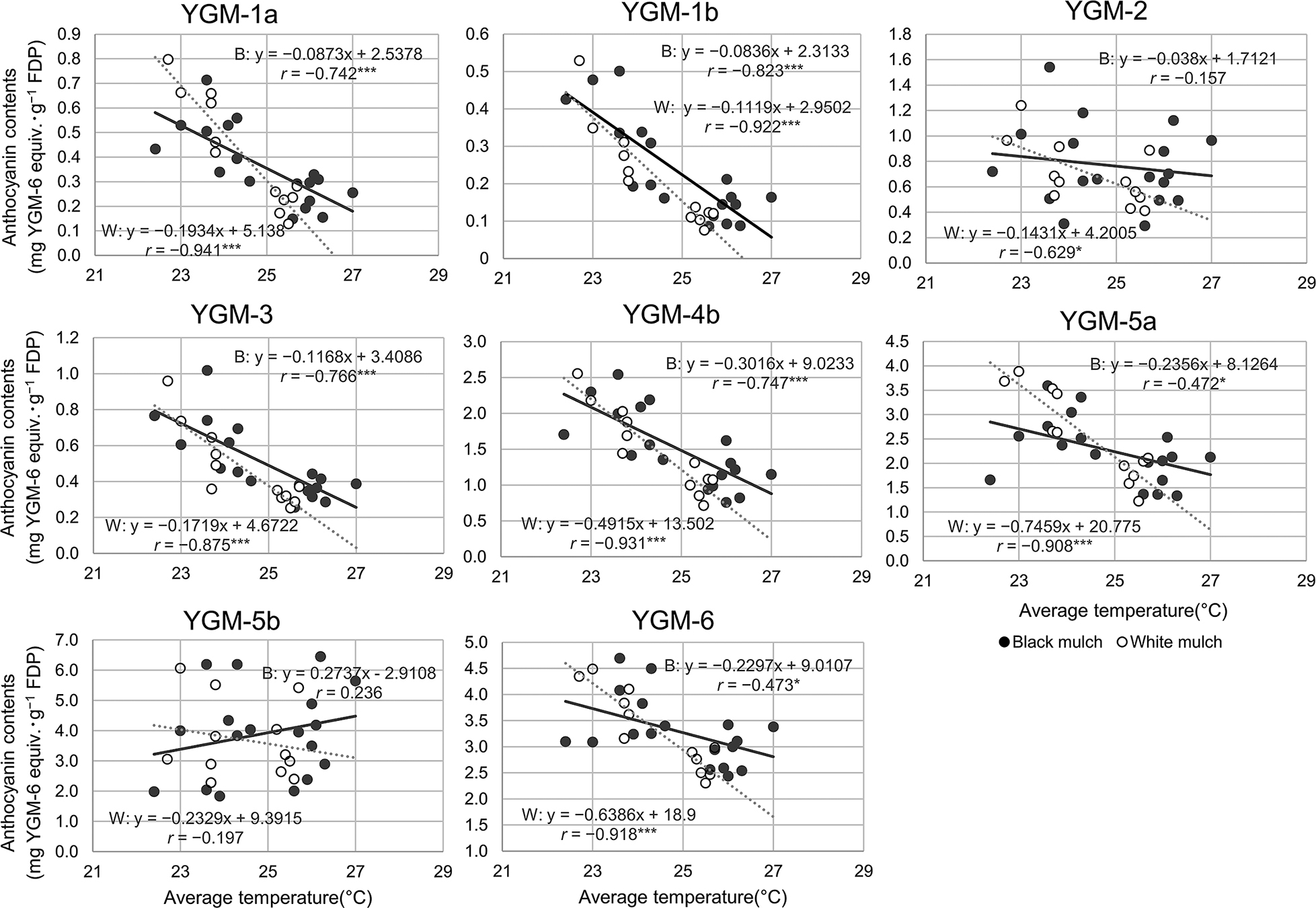
Relationship between average soil temperature and anthocyanin content during the cultivation period. Black dots: black mulch-film; gray dots: white mulch-film. The equations on the W and B in each graph indicate correlation coefficients r for white and black mulch-films, respectively. The vertical axis indicates anthocyanin content the horizontal axis indicates soil temperature. Asterisks (* and ***) indicate significance at P < 0.05 and P < 0.001, respectively.
We investigated the effect of cultivating the purple sweet potato variety ‘Murasakimasari’ in the same soil under different temperature conditions. Although there are reports of relationships between soil temperature and starch content (Noda et al., 2001), and temperature and morphogenesis in sweet potatoes (Wijewardana et al., 2018), very few reports have focused on the relationship between soil temperature and anthocyanins. The average soil temperature of the white mulch was lower than that of the black mulch, but a change of 0.5°C or more appeared only in the test plots planted in May (Table 1). It is assumed that the large difference in soil temperature due to the mulch appeared only for one month after planting until the sweet potato foliage covered the mulch. In all test plots, the soil temperature after the foliage covered the mulch showed about the same variation between white and black mulch. Therefore, the average temperature between black and white mulch in May, which is the coldest month in the planting season, showed the largest difference. However, the difference in temperature due to the mulch at the time of planting in May did not affect the tuberous root yield (Tables 2 and 3). The low soil temperature for the first month after planting in May seems to have had little effect on the tuberous root yield. However, when comparing the 120-day cultivation periods, the tuberous root yield tended to decrease in the order of planting test plots: May < Jun < Jul, so it is assumed that a high temperature during the first month after planting has a negative effect on tuberous root yield. Furthermore, when comparing GR values by planting month, significant differences were observed for May > Jun > Jul planting (Table 4). This may be because the seedlings planted in July are less likely to establish due to high temperatures despite high humidity, and that in June, the rainy season brings more moisture to the soil, which makes the roots weaker and more prone to growth. Therefore, it was suggested that planting in May, when soil temperature and humidity were lower, would result in robust initial growth, including root establishment, and contribute to increased tuberous root yield. In recent years, due to global warming, early planting in April and even March is sometimes done. We would like to study the impact of these changes in the future. In addition, heavy rainfall in Jun and intense solar radiation in Jul can easily be assumed as growth stresses as cultivation environmental factors that affect tuberous root yields, and these factors should also be considered in the future. The suppression of high temperature by white mulch alone could not significantly improve the tuberous root yield. The most effective way to increase the tuberous root yield was to extend the number of cultivation days.
It has been reported that anthocyanins in potatoes cultivated under controlled temperature conditions showed less pigment accumulation at high temperature differences of ± 5°C (Liu et al., 2019). In sweet potatoes, the difference in average soil temperature between May–Oct and Jun–Nov, under cultivation for 150-days in white mulch, was only 1.5°C, but a significant difference was observed in the total anthocyanin content. For the same cultivation period, the anthocyanin content was significantly higher with the lower average soil temperature than that the higher average temperature (Table 5). This suggests that sweet potato anthocyanin synthesis and accumulation is influenced by cultivation temperature. The anthocyanin components YGM-1–6 showed a significant negative correlation with soil temperature, except for YGM-2 and -5b (Figs. 1, 2, and 4). In YGM-2 and -5b, a sophorose residue bound at 3 position is not acylated, suggesting that these are precursors of other acylated anthocyanins. Many enzymes have been reported to be involved in anthocyanin biosynthesis (Li et al., 2019; Sasaki et al., 2014). In the anthocyanin biosynthesis pathway, YGM-2 and -5b may simply accumulate due to their low enzymatic activity to generate the next product in an enzymatic reaction. Alternatively, it is speculated that YGM-2 and -5b are accumulated due to insufficient supply of the bound organic acid. YGM-5b is the most abundant anthocyanin in ‘Murasakimasari’ that impacts the total anthocyanin content. Similarly, it was observed that there was no correlation between total anthocyanin content and soil temperature in black mulch due to the lack of correlation between YGM-5b content and soil temperature (Figs. 2, 3, and 4). In addition, white mulch is more effective than black mulch in suppressing rises soil temperature; it is speculated that the temperature of white mulch is lower than that of black mulch and that this contributed to the resulting increase in anthocyanin content. These results support previous observations of changes in anthocyanin content depending on the cultivation area on the basis of soil temperature (Ishiguro et al., 2022). However, this study did not consider factors such as water stress that could cause an increase or decrease in anthocyanins as reported in other plants (Castellarin et al., 2007; Hinojosa-Gómez et al., 2020). Therefore, it is essential to investigate such aspects in further studies.
Higher tuberous root yields and higher anthocyanin contents resulted in better anthocyanin yield, but tuberous root yield and anthocyanin contents varied depending on soil temperature (Tables 2, 5, and 6). Therefore, a coefficient of variation (CV) value was calculated from all data and used to test whether tuberous root yield or anthocyanin content had more influence on anthocyanin yield. The results showed that tuberous root yield (CV = 0.36) had a higher CV than anthocyanin content (CV = 0.29). In all test plots, tuberous root yield was more variable than anthocyanin content, indicating a greater influence on the contribution to anthocyanin yield.
This study investigated the effect of soil temperature on tuberous root yield and anthocyanin content in purple sweet potato. Changes in cultivation periods and the use of white mulch in addition to black mulch created variations in the average soil temperature during cultivation. Soil temperature in white mulch cultivation was lower than that in the black mulch cultivation, indicating the superiority of white mulch for tuberous root yield and GR in the Jun–Oct test plots. Anthocyanin content was negatively correlated with soil temperature in white mulch cultivation; white mulch may have facilitated an increase in anthocyanin content as the soil temperature was lower than that of black mulch. Therefore, white mulch is ideal for purple sweet potato cultivation; moreover, maximization of anthocyanin yield is achieved with an increase in tuberous root yield following long-term cultivation.
The authors thank Dr. Yoichi Nishiba (KARC, NARO) for kindly providing the YGM-6 standards, Dr. Masaru Tanaka (KARC, NARO) for helpful suggestions on the manuscript, and Ms. Ayumi Yonetsu for technical assistance.