2023 年 92 巻 3 号 p. 299-307
2023 年 92 巻 3 号 p. 299-307
Melon Fusarium wilt, caused by Fusarium oxysporum f. sp. melonis (Fom), is one of the most prevalent fungal diseases affecting melon (Cucumis melo L.). We aimed at finding an effective resource for breeding cultivars tolerant to Fom race 1.2, which includes pathotypes that cause yellowing (race 1.2y) and wilting (race 1.2w). We screened 294 melon accessions that originated mainly in Africa and Asia for tolerance to race 1.2y. The highest level of tolerance was observed in the Indian accession PI124550; one of the five PI124550 plants tested showed no disease symptoms. From this plant, a tolerant inbred line, YR01, was developed after seven rounds of selfing combined with selection for tolerance to race 1.2y. YR01 had a high level of tolerance not only to race 1.2y, but also to race 1.2w. It was more tolerant to race 1.2y than the tolerant reference control ‘Isabelle’ and was equally tolerant to race 1.2w. Analysis of F1 hybrids and an F2 population developed by crossing YR01 with susceptible ‘Earl’s Favorite Harukei 3’ suggested that YR01 had multiple recessive and dominant (or codominant) genes for tolerance to race 1.2y and one dominant gene for tolerance to race 1.2w. These results indicate that PI124550 and its derivative YR01 are a promising breeding material with novel genes conferring practically useful tolerance to both pathotypes of race 1.2.
Melon Fusarium wilt is caused by a soilborne fungal pathogen, Fusarium oxysporum Schlechtend f. sp. melonis Sny & Hans (Fom). It is one of the most economically damaging plant diseases worldwide (Erzurum et al., 1999; Namiki et al., 1998; Schreuder et al., 2000; Zuniga et al., 1997). The pathogen penetrates the host plant through the root system, invades vascular elements, and causes wilting and plant death (Gordon and Martyn, 1997). Four physiological races of the pathogen—0, 1, 2, and 1.2—have been identified according to their ability to infect differential melon genotypes (Risser et al., 1976). Resistance to races 0 and 2 or races 0 and 1 is conferred by a single dominant gene, Fom-1 or Fom-2. Race 1.2 can overcome these two resistance genes and has two pathotypes, 1.2y, which causes yellowing, and 1.2w, which causes wilting (Risser et al., 1976). Several accessions resistant to races 0, 1, and 2 have been identified (Alvarez et al., 2005; Pitrat et al., 1996), and cultivars resistant to these races have been developed. However, little progress has been made in the development of new cultivars with resistance or high tolerance to race 1.2.
Melon production in Japan has been negatively affected by race 1.2 since 1989 (Iwata et al., 1994; Tanaka and Tamura, 1997; Usuki et al., 2006). Since all Japanese F1 hybrid cultivars are susceptible to race 1.2, Fusarium wilt is controlled by grafting on moderately or weakly tolerant rootstocks. However, even rootstock cultivars are often damaged by race 1.2 at low temperatures or high fungal density in soil. Soil disinfection is used to suppress the spread of Fusarium wilt and to achieve high fruit yield and good quality, but this requires time, labor, materials, training, and experience to optimize the frequency and timing of disinfectant application. Genetic resistance is the ideal means of overcoming Fusarium wilt caused by race 1.2. It is of great importance to identify breeding materials with resistance or high tolerance to race 1.2y to develop new resistant cultivars.
Risser and Rode (1973) reported that a few accessions from Far East Asia, such as ‘Ogon-9’ and ‘Kogane Nashi Makuwa’ (horticultural group Makuwa) are tolerant to race 1.2. Accessions tolerant to race 1.2 have also been reported (Chikh-Rouhou et al., 2010, 2021; Herman and Perl-Treves, 2007; Oumouloud et al., 2009). Tolerant rootstock cultivars ‘Dodai No. 1’ and ‘Dodai No. 2’ have been bred in Japan by accumulating tolerance genes derived from several accessions of the Makuwa and Conomon groups (Hirai et al., 2002). The tolerance of these accessions is considered to be controlled by several recessive genes; Perchepied et al. (2005) detected nine recessive QTLs for the tolerance to race 1.2 in ‘Isabelle’, a partially tolerant cultivar developed from ‘Ogon-9’. The fruit traits of these accessions differ considerably from those of commercial F1 hybrids of netted melon (the Reticulatus group) in Japan. Therefore, it is very difficult to introduce all recessive genes from the above tolerant accessions into commercial F1 cultivars without marker-assisted selection (MAS).
The ideal breeding materials should have robust complete resistance or high tolerance controlled by one or two genes. This study aimed at screening diverse melon accessions for high tolerance to race 1.2y, which occurs over a wider region than race 1.2w, and to evaluate the usefulness of selected tolerant accessions in practical melon breeding. We identified an accession highly tolerant to race 1.2y and developed the inbred tolerant line YR01 by repeated selfing and phenotyping. Then we compared the tolerance of YR01 and other tolerant cultivars. We also performed crosses to clarify the manner of inheritance of the tolerance genes, because this knowledge is needed for the systematic breeding of new tolerant F1 hybrid cultivars.
We grew 294 accessions of Cucumis melo L. that originated mainly in Africa and Asia (Tables 1 and S1); Africa has been postulated as the place of the species’ origin and initial evolution, and India is likely a secondary center of melon diversification (Bates and Robinson, 1995; Robinson and Decker-Walters, 1997). Four cultivars were used as references: ‘Earl’s Favorite Harukei 3’ (EF3), susceptible to all Fom races; ‘Charentais Fom-1’, resistant to races 0 and 2; ‘Charentais Fom-2’, resistant to races 0 and 1; and ‘Isabelle’, resistant to races 0, 1 and 2 and tolerant to race 1.2. Japanese commercial rootstock cultivars RC1, RC2, and RC3 were also used.
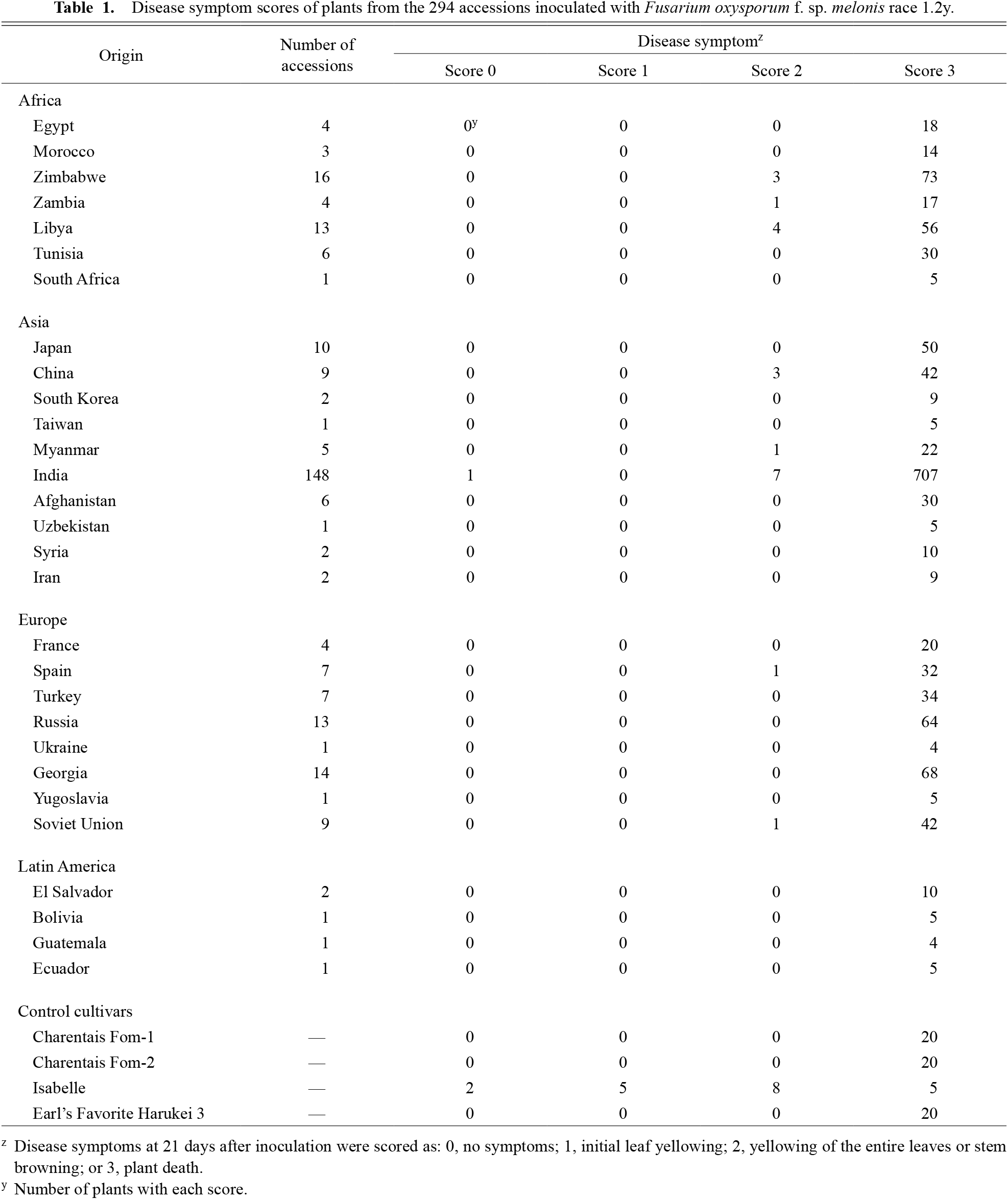
Disease symptom scores of plants from the 294 accessions inoculated with Fusarium oxysporum f. sp. melonis race 1.2y.
Five Fom strains from the major cultivation areas in Japan were used: Mel02005 (race 0, JCM 9286), Fom17-S3 (race 1, MAFF 242351), Mel02010 (race 2, JCM 9288), Fom142-S1 (race 1.2y, MAFF 242350), and Iba02001-S12 (race 1.2w, MAFF 242350). The JCM accessions were provided by the Microbe Division, RIKEN BioResource Research Center, Japan, and the MAFF accessions by the Research Center of Genetic Resources, National Agriculture and Food Research Organization (NARO), Japan.
Preparation of spore suspensionFungal strains were grown on 300 mL flasks containing 100 mL of potato dextrose broth, and cultured on a rotary shaker at 100 rpm at 24°C. After seven days, the cultures were filtered through doubled gauze, and the resulting filtrate was used as a suspension. Spore concentrations of the suspension were determined by a hemocytometer and adjusted to 1 × 107 spores·mL−1 by adding sterile distilled water. Tween 20 was added at 0.02% (v/v) to promote dispersibility of the spore suspension.
Screening melon accessions for tolerance to race 1.2y and selection of tolerant plantsSeeds of each accession were sown in a tray (500 mm length, 370 mm width and 110 mm height) containing seedling-raising soil and grown in a growth chamber at 25°C (16 h day/8 h night) until inoculation. Four or five seedlings with three or four true leaves were tested per accession. Seedlings were cut between the first and second true leaves (Fig. 1A). The cutting was inserted into a hole poked in soil in a pot (42 mm long, 42 mm wide and 45 mm high). Inside the growth chamber, the pots were placed in a clear box with a lid (500 mm length, 370 mm width and 220 mm height) to maintain high humidity. After one week, cuttings with regenerated roots were acclimatized by removing the lid of the box and were grown as Fusarium-free clones.

Method for screening and selection of plants tolerant to Fusarium oxysporum f. sp. melonis.
The basal part of each seedling (Fig. 1A-b) was removed from the tray, and the roots were rinsed in tap water, wiped, and soaked in the spore suspension for ~10 s. Each inoculated seedling was replanted into a plastic pot and grown in a growth chamber under a 16 h day/8 h night photoperiod at 24°C day/23°C night. Disease symptoms were evaluated at 21 days after inoculation and scored by eye as 0, no symptoms; 1, initial leaf yellowing; 2, yellowing of the entire leaves or stem browning; and 3, plant death (Fig. 2A). Accessions including plants with scores 0 or 1 were considered tolerant or resistant.

Typical disease symptoms caused by Fusarium oxysporum f. sp. melonis and scales for symptom evaluation: (A) yellowing by race 0, 1, 2, and 1.2y, (B) wilting by race 1.2w.
Since one of five seedlings from one accession was tolerant to race 1.2y, Fusarium-free clones (S0 generation) regenerated by cutting five seedlings (Fig. 1A-a) were transplanted to a greenhouse and self-pollinated (Fig. 1B) to obtain S1 seeds. To select additional tolerant S1 plants from the accession, plants were grown from 26 original seeds (P6–P31) of the same accession stored at Okayama University without inoculation and were self-pollinated. All 31 S1 lines (29 or 30 plants) were inoculated when the cotyledons were fully unfolded (Fig. 1C); plants were visually evaluated for disease symptoms (score 0–3) as described above. Disease severity (DS) was calculated as (Σ (number of plants with score × corresponding score value)/(maximum score value × total number of plants)) × 100. Resistance and tolerance of each line were determined based on the DS values; resistant (DS = 0), highly tolerant (0 < DS ≤ 30), moderately tolerant (30 < DS ≤ 60), weakly tolerant (60 < DS ≤ 90), and susceptible (90 < DS). The S0 lines with low DS were selected, and 70 S1 plants derived from each selected S0 line were subjected to a screening test (Fig. 1A). Six or seven S1 plants with a score of 0 or 1 were randomly selected, and their clones were self-pollinated to obtain S2 seeds (Fig. 1B). Selection of the lowest-DS line and generation advancement (self-pollination of plants from the lowest-DS line) were repeated until the S7 generation. A fixed S7 line was named YR01 and its tolerance was tested in detail using the following methods.
Characterization of YR01 tolerance to race 1.2yTo investigate which parts of the plant result in tolerance, the tolerance of YR01 to race 1.2y was evaluated using three different inoculation methods. Seedling roots (Fig. 3A), the cut end of cuttings (Fig. 3B), and roots of clones (Fig. 3C) of seedlings with unfolding cotyledons were soaked in spore suspension. After using a cutting method (Fig. 3B), hypocotyls soaked in the spore suspension were replanted into plastic pots. The pots were kept in a clear box with a lid to maintain high humidity, and the lid was removed after 10 days for acclimatization. These tests were carried out using seven seedlings with six replications. The disease symptoms were evaluated one week after all ‘EF3’ seedlings had died, and DS was calculated.
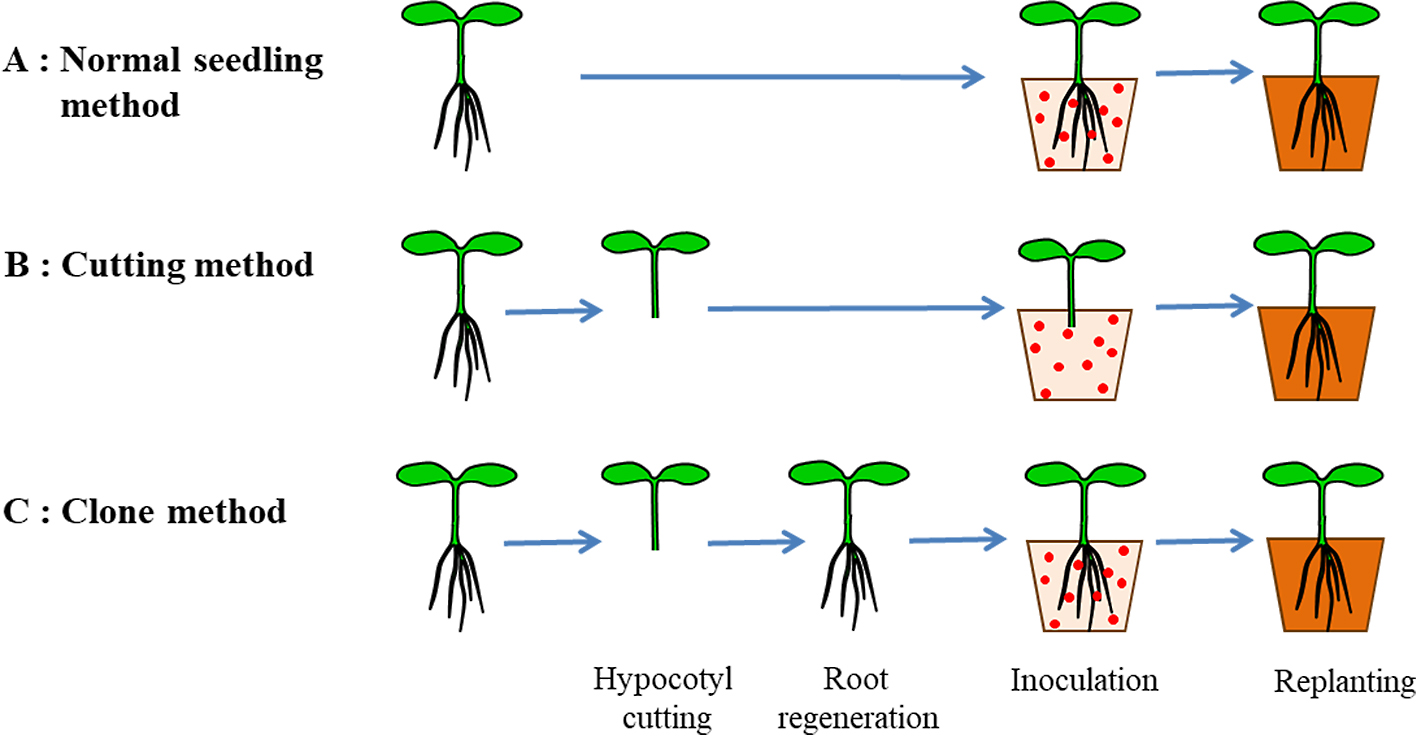
Three methods for inoculating Fusarium oxysporum f. sp. melonis.
Responses of YR01 to different pathotypes of Fom race 1.2 and different Fom races were evaluated together with those of the four reference cultivars. For races 0, 1, and 2, the disease symptoms were scored as for race 1.2y (Fig. 2A), while those for 1.2w were scored as 0, no symptoms; 1, initial wilting; 2, wilting of the entire plant or stem browning; and 3, plant death (Fig. 2B). In order to confirm whether the tolerance of YR01 is exhibited at different temperatures, the response of YR01 to the two pathotypes of race 1.2 was evaluated by the normal seedling method (Fig. 3A) at two different temperature conditions (21°C 16 h day/19°C 8 h night and 24°C 16 h day/23°C 8 h night) and compared with those of ‘Isabelle’, ‘EF3’, and the three rootstock cultivars. Seven seedlings with six replications were used.
Tolerance of F1 and F2 progeny to Fom race 1.2To clarify the mode of inheritance of the tolerance, the responses to the two pathotypes of races 1.2 were examined using reciprocal F1s between YR01 and ‘EF3’ (susceptible) by the normal seedling method (Fig. 3A), and compared with those of their parents, ‘Isabelle’, and the three rootstock cultivars. Inoculation tests were carried out using seven seedlings with six replications. We also examined the responses of 197 or 198 plants of the F2 generation (YR01 × EF3) to two pathotypes of race 1.2. F2 plants with scores 0 or 1 were classified as tolerant and those with scores 2 or 3 as susceptible.
Among 294 accessions tested, all except one proved to be susceptible (score 2 or 3; Table 1). The exception was PI124550, of Indian origin; it included a tolerant plant (P1, score 0) and susceptible plants (P2–P5, score 3). Among the S0 lines P1–P31, two lines were moderately tolerant (DS 51.7 for P1 and 56.7 for P15), two lines were weakly tolerant (DS 76.7 for P6 and 78.9 for P24), and 27 lines were susceptible (DS > 90). This result suggested that approximately 13% of the original PI124550 seeds have a tolerance gene(s) for race 1.2y. Fruit appearance was highly diverse in this accession (Fig. 3A).
Breeding of a fixed line with tolerance genes to race 1.2yTo obtain fusarium-free clones of tolerant S1 plants, 70 additional S1 seeds from each of the four selected S0 lines (P1, P6, P15, and P24) were sown and disease symptoms were evaluated as in the screening test (Fig. 1A). From each S0 line, six or seven S1 plants with score 0 or 1 were randomly selected, and their clones were self-pollinated to obtain S2 seeds (Fig. 1B). The DS of each S1 line was evaluated using 22 to 24 S2 plants (Fig. 1C) to select two or three lines, from each of which 70 S2 seeds were sown and disease symptoms were evaluated (Fig. 1A). Three or four S2 plants with score 0 were randomly selected and their clones were self-pollinated to obtain S3 seeds (Fig. 1B). The DS of each S2 line was evaluated using 14 S3 plants (Fig. 1C). In the S2 line, a significant difference in DS was found between the P1 and P15 progeny and those of P6 and P24 (Tukey’s test, P < 0.05); P1 progeny (subline P1-1-1) had the lowest DS (23.8; Fig. S1). The proportion of tolerant plants (those with score 1 or less) was 92.9%, indicating the tolerance was very high. The external fruit appearance of P1 progeny also became almost uniform in the S2 generation (Fig. 4B). Plants grown from five randomly selected S3 seeds of P1 progeny were self-pollinated without inoculation. The tolerance of the five S3 lines was evaluated by the DS of 20 S4 plants (Fig. 1C), and one plant with the lowest DS was selected. After the S3 generation, selection of the lowest-DS line (evaluated using 19 or 20 plants, Fig. 1C) and generation advancement were repeated until the S7 generation, and a tolerant inbred line named YR01 was developed (Figs. S1 and 4C).

Fruit appearance of line PI124550. Generations: (A) S0, (B) S2, (C) S6.
The DS of ‘EF3’ (susceptible to race 1.2y) was 100.0 with all three inoculation methods (Table 2); those of four tolerant cultivars (‘Isabelle’, RC1, RC2, RC3) were 45.0–66.7 (normal seedling method), 23.3–61.7 (cutting method), and 36.7–50.0 (clone method). That of YR01 was the lowest with the normal seedling method (18.3) and the clone method (16.7); surprisingly, it was 100 with the cutting method.
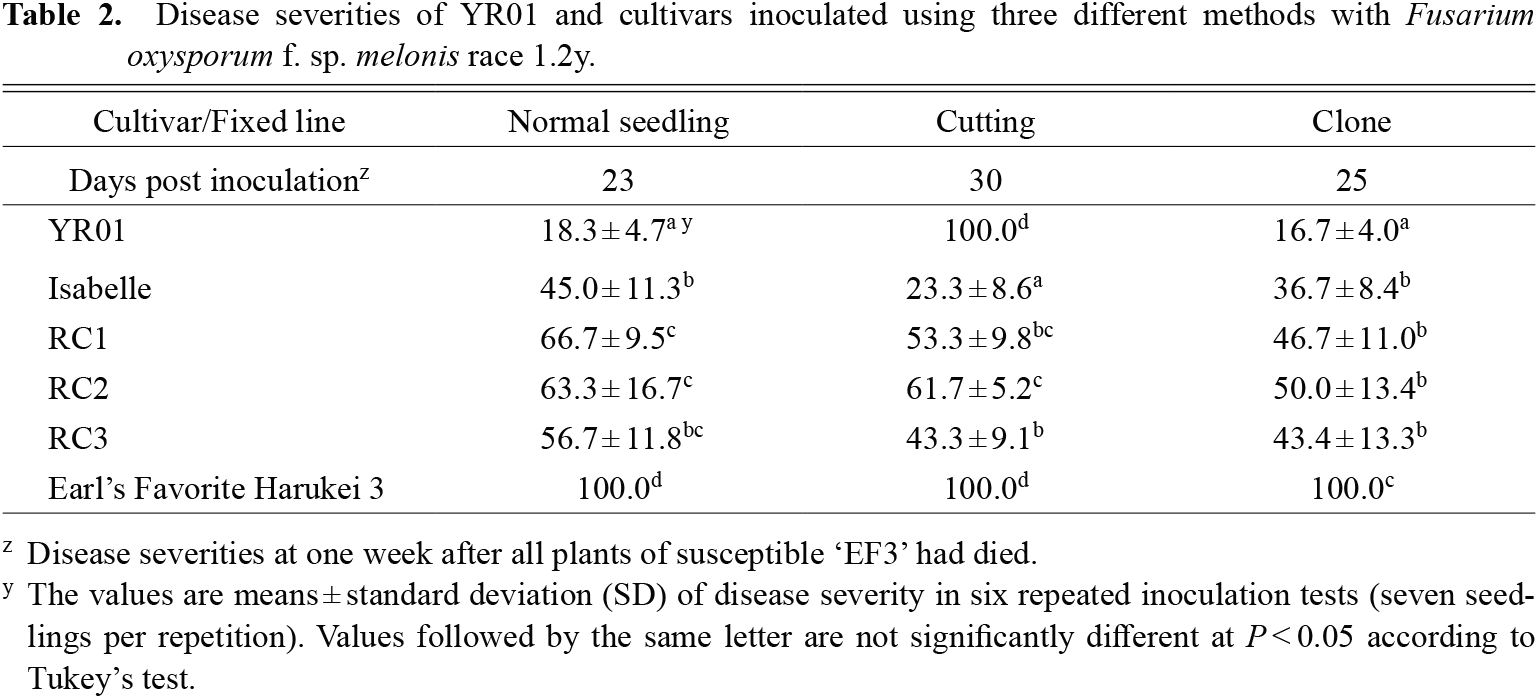
Disease severities of YR01 and cultivars inoculated using three different methods with Fusarium oxysporum f. sp. melonis race 1.2y.
The DS values of ‘EF3’ (susceptible to races 0, 1, 2, 1.2y and 1.2w) were ≥ 98.4, while those of ‘Charentais Fom-1’ against races 0 and 2 and of ‘Charentais Fom-2’ against races 0 and 1 were 0.0 (Table 3). The DS of ‘Isabelle’ (carrying Fom-1 and Fom-2) was 0.0 against races 0, 1, and 2. ‘Isabelle’ was also moderately tolerant to race 1.2y (DS = 56.5) and highly tolerant to race 1.2w (DS = 13.1). YR01 was highly tolerant to races 1.2y (DS = 11.9), 1.2w (DS = 8.3), and races 0 (DS = 28.6) and moderately tolerant to race 1 (DS = 32.5). The DS of YR01 against race 2 was 100.0, indicating that YR01 is susceptible to this race. YR01 proved to be more tolerant to race 1.2y than ‘Isabelle’ and equally tolerant to race 1.2w.

Disease severities of YR01 and cultivars inoculated with four Fusarium oxysporum f. sp. melonis races.
All cultivars except ‘EF3’ showed higher DS at 19°C night/21°C day than at 23°C night/24°C day, and there were significant differences between cultivars/lines in each condition (Table 4). As expected, nearly all ‘EF3’ plants were wilted by both races 1.2y and 1.2w under both temperature conditions. ‘Isabelle’ was highly tolerant to race 1.2w under both conditions, but moderately tolerant to race 1.2y at 23°C night/24°C day and weakly tolerant at 19°C night/21°C day. The tolerance of the three rootstock cultivars was nearly equal to that of ‘Isabelle’ against race 1.2y and was equal or lower against race 1.2w. YR01 had the highest tolerance to both 1.2y and 1.2w under both temperature conditions.

Disease severities of YR01 and cultivars inoculated with Fusarium oxysporum f. sp. melonis race 1.2 at two temperature conditions.
The tests showed DS values against race 1.2y of 14.3 for YR01, 100 for ‘EF3’, and 85.7 and 84.9 for the reciprocal F1 hybrids (Table 5); there was a significant difference between the F1 hybrids and the parental lines, but not between the reciprocal F1s. This result indicates that the tolerance of YR01 to race 1.2y is controlled by nuclear recessive gene(s). The F1 hybrids were less tolerant than ‘Isabelle’ and the rootstock cultivars. The tests showed DS values against race 1.2w of 10.3 for YR01, 90.5 for ‘EF3’, and 22.2 and 21.4 for the F1s (Table 5); there was a significant difference between the F1 hybrids and EF, but not between the F1s and YR01 and between the reciprocal F1s. This result indicated that the tolerance to race 1.2w is controlled by nuclear gene(s) and is inherited dominantly. The tolerance of the F1 hybrids was the same level with ‘Isabelle’ and superior to those of the rootstock cultivars. In F2, 116 plants were tolerant and 81 were susceptible to race 1.2y, and 145 were tolerant and 53 were susceptible to race 1.2w. The segregation ratio in race 1.2y did not fit the 3:1 ratio expected for a single recessive gene (χ2 = 26.4, P = 2.72×10−7), but that in race 1.2w did fit the 3:1 ratio expected for a single dominant gene (χ2 = 0.48, P = 0.62).

Disease severities of F1 hybrids of YR01 and ‘Earl’s Favorite Harukei 3’ and cultivars inoculated with Fusarium oxysporum f. sp. melonis race 1.2.
We screened 294 accessions of melon genetic resources and selected a novel material (one of the five plants of accession PI124550, of Indian origin) highly tolerant to Fom race 1.2, including pathotypes 1.2y and 1.2w. To obtain selfed seeds of the tolerant plant, we established a stable and highly efficient propagation method using cuttings, and obtained an uncontaminated/uninoculated clone of each plant. In this system, the original plant was used for primary evaluation, and the tolerance was accurately evaluated using selfed seeds of the uncontaminated clone (progeny test, Fig. 1). Moderately tolerant plants P1 and P15 and weakly tolerant plants P6 and P24 were selected from 31 plants of PI124550. Our data suggest that PI124550 is heterogeneous in terms of tolerance QTLs and that P1 and P15 had more QTLs than other PI124550 plants.
An important point to consider is the inoculum concentration. In previous studies, seedlings were inoculated by dipping them into a suspension of 1 × 105 to 3 × 106 spores·mL−1 (Chikh-Rouhou et al., 2010, 2021; Herman and Perl-Treves, 2007; Oumouloud et al., 2009), whereas the concentration used here was 1 × 107 spores·mL−1. This biotic stress was extremely severe, and nearly half of the plants were infected even in the tolerant cultivar ‘Isabelle’. Under such severe selection, most plants of the 294 accessions were susceptible, while only one plant (P1) of PI124550 was highly tolerant (score 0; Table 1). This approach enabled us to efficiently evaluate the tolerance of many plants and to select resistant plants. Selection in the subsequent generations was also effective, and the DS decreased from 51.7 in the S0 generation to 16.7 in S6 (Fig. S1).
Several tolerant cultivars or lines have been bred by using germplasms of the Makuwa and Conomon groups (Ficcadenti et al., 2002; Hirai et al., 2002). According to Fujishita (1983) and Akashi et al. (2002), melon groups can be classified into large-seed types (seed length ≥ 9.0 mm) and small-seed types (seed length < 9.0 mm). Most accessions of the Cantalupensis and Inodorus melon groups are classified as large-seed types and those of the Agrestis, Makuwa, and Conomon groups as small-seed types. The tolerance of ‘Isabelle’ and other tolerant lines was derived from genetic resources of the latter groups. In contrast, the average length of 10 seeds in each S1 line exceeded 10 mm, indicating that PI124550 does not belong to Makuwa or Conomon. Thus, the tolerance QTLs of PI124550 may differ from those of tolerant cultivars and lines developed to date. This hypothesis is supported by the results obtained by three different inoculation methods (Table 2). The tolerant cultivar ‘Isabelle’ and three rootstock cultivars had the same level of tolerance regardless of the inoculation method. However, YR01 had the highest tolerance using the normal seedling and clone methods, but no tolerance using the cutting method. This result clearly shows that the tolerance of YR01 works only in the root system. Therefore, its tolerance mechanism differs from that of other tolerant cultivars and lines.
YR01 was tolerant not only to race 1.2y but also to race 1.2w and other races (0 and 1) (Table 3). ‘Kogane Nashi Makuwa’, C-211, ‘Shiro Uri Okayama’, TUN-5, and TUN-26 are also reportedly tolerant to both race 1.2 pathotypes (Chikh-Rouhou et al., 2010, 2021). However, not all cultivars are tolerant to both; ‘ANC-57’, ‘Baza’, ‘Encin 4078’, ‘Korcxa’, ‘Mollerusa-7’, ‘Piñonet’, and Rayado are tolerant to race 1.2w only (Chikh-Rouhou et al., 2010). YR01 was more tolerant to both pathotypes than ‘Isabelle’ and commercial rootstock cultivars under two temperature conditions (23°C night/24°C day and 19°C night/21°C day; Table 4), suggesting that YR01 carries more effective QTLs. In melon production areas in Japan, even plants grafted onto tolerant rootstock cultivars are often severely damaged depending on environmental conditions. In both race 1.2y and 1.2w, the tolerance of all cultivars and lines tended to decrease under 19°C nighttime/21°C daytime conditions. Especially in race 1.2w, the tolerance of rootstock cultivars decreased greatly (Table 4).
In contrast, YR01 was highly tolerant regardless of temperature. Thus, YR01 is a novel material potentially useful for practical breeding. However, its tolerance to other races was not uniform: YR01 was susceptible to race 2 and was partially tolerant to races 0 and 1 (Table 3). Therefore, we conclude that YR01 does not have Fom-1. The partial tolerance to races 0 and 1 may be caused by sequence polymorphism at Fom-2, or by a gene other than Fom-2. Therefore, the tolerance QTL(s) of YR01 should be combined with Fom-1 and Fom-2 to achieve robust tolerance for production in areas where various Fom races cause serious economic damage.
Our progeny test provided an insight into the inheritance of the tolerance QTLs. No significant difference in disease severity was detected between reciprocal F1 hybrids (Table 5), indicating that the tolerance of YR01 to each pathotype is of nuclear origin. Tolerance to race 1.2y is controlled by multiple genes in YR01, as in ‘Isabelle’, because F2 segregation did not fit the 3:1 ratio for a single-gene model. The level of tolerance to race 1.2y was much lower in F1 hybrids than in YR01 and higher than in ‘EF3’. Therefore, the tolerance of YR01 to race 1.2y is controlled by multiple QTLs and inherited recessively. It was also lower than that of commercial rootstock cultivars and would not be practically useful. Therefore, it is necessary to introduce tolerance QTLs into both parents in the breeding of a new tolerant F1 cultivar. In contrast, the level of tolerance to race 1.2w did not differ significantly between F1 hybrids and YR01, indicating that the tolerance was a completely dominant trait. Segregation of tolerance to race 1.2w in the F2 generation fit the 3:1 ratio well. These results suggest that the tolerance of YR01 to race 1.2w is monogenic and can be easily transferred to parental lines through a backcross breeding program. The tolerance of ‘Isabelle’ to race 1.2 is controlled by nine recessive QTLs (Perchepied et al. 2005). On the other hand, the nearly complete resistance of the breeding line BIZ is conferred by two complementary recessive genes (Herman and Perl-Treves, 2007; Herman et al., 2008), but whether this line is resistant to race 1.2y or 1.2w is unknown. We consider the tolerance of YR01 to race 1.2 to be caused by QTLs that are clearly different from those of ‘Isabelle’ and BIZ, indicating that YR01 has several novel tolerance genes.
In conclusion, PI124550 and its derivative YR01 are a promising new source of tolerance to both pathotypes of race 1.2. The challenge ahead is to identify the QTLs involved. We anticipate that the results reported here will contribute not only to the development of new melon cultivars highly tolerant to race 1.2, but also to further analyses aimed at understanding the genetic mechanisms of tolerance, and thereby the development of a marker-assisted selection system for this trait.
We thank Takashi Ogawara at the Horticultural Research Institute, Ibaraki Agricultural Center, for technical advice and assistance.