2023 年 92 巻 3 号 p. 216-226
2023 年 92 巻 3 号 p. 216-226
Abiu is a tropical fruit with many beneficial bioactive compounds. However, its economic value is limited by a short shelf life and rapid browning. This study evaluated the effect of ascorbic acid on extending abiu fruit shelf life during storage. The fruit was soaked in ascorbic acid solutions of 0 mM (control), 5 mM (AA5), and 10 mM (AA10) for 10 minutes, air-dried, then stored in an ambient room (28 ± 1°C and 80 ± 5% RH) for 12 days. The results indicated that the 10 mM exogenous ascorbic acid treatment increased abiu fruit shelf life up to 12 days, nine days longer than the control. Abiu fruit, after the AA10 treatment, underwent a 1.4-fold lower weight loss than the control. In addition, on day 12 of storage, the browning of fruit with the AA10 treatment were 8 and 11% on the peel and pulp, respectively. The climacteric peak of abiu fruit in the AA10 treatment occurred on day 8 of storage, three days later than the control. The AA10 treatment also maintained vitamin C content and fruit firmness. Thus, the AA10 treatment effectively extended shelf life and maintained abiu fruit quality.
Fruits reduce risks of diseases, including cancers and cardiovascular disease, support increased fruit consumption (Isabelle et al., 2010). Abiu fruit is a tropical fruit that contains important bioactive compounds such as 1-(2-Hydroxyethyl)-1,2,4-triazole, 5-hydroxy methyl furfural, 1-methyl-5-fluorouracil, and trans-geranylgeraniol (Arif et al., 2021), known anti-cancer agents (Al-Soud et al., 2004; Cristensen et al., 2019; Kumar and Periyasamy, 2016; Zhao et al., 2013). Additionally, the abiu fruit is reported to have anti-microbial properties (Abreu et al., 2019), inhibit acetylcholinesterase (Fernandez et al., 2020), and exhibit anti-cancer activity (Veeramani et al., 2021). However, abiu fruit suffer from a short shelf life, and rapidly turn brown (Arif et al., 2022).
The abiu fruit is climacteric, with a steep respiration peak and high levels of ethylene production, which limits its shelf life (Arif et al., 2022). The shelf life of harvested fully ripe abiu fruit is roughly six days, and, after two days of storage, it undergoes a significant increase in browning index (Arif et al., 2022). These qualities limit the market distribution of abiu fruit. Furthermore, peel browning is a major problem for fruit storage and processing. Browning is reported to be responsible for more than 50% of fruit industry losses (Jaeger et al., 2018). Therefore, postharvest treatments are required to maintain fruit quality and increase shelf life.
Ascorbic acid is an additive that maintains both fruit quality and extends shelf life. Coating treatments containing ascorbic acid delayed 1-aminocyclopropane-1-carboxylic (ACC) production in guava (Martinez-Ortiz et al., 2019), and diminished weight loss in strawberries (Sogvar et al., 2016). In addition, pre-harvest ascorbic acid treatments are reported to inhibit the polygalacturonase (PG) enzyme and maintain grape firmness (Lo’ay and El-Boray, 2018). Moreover, ascorbic acid is an antioxidant widely used to prevent fruit browning in strawberries (Sogvar et al., 2016), mangoes (Lo’ay and Ameer, 2019), and guava (Azam et al., 2021).
According to Moon et al. (2020), there are three main mechanisms behind the ascorbic acid inhibition of browning: 1) ascorbic acid acts as an antioxidant, promoting o-quinone regeneration and limiting polymerization into brown pigments; 2) ascorbic acid binds to histidine residues which are PPO catalytic by-products, and reduces the turnover of oxidized phenols induced by PPO enzymes; 3) ascorbic acid, is a weak acid, and its accumulation can lower the pH of the cytosol, also reducing oxidase enzyme activities.
The influence of ascorbic acid on postharvest fruit quality and abiu fruit browning response during storage is not yet evaluated. Therefore, this study examines the effect of ascorbic acid on extending abiu fruit shelf life during storage in terms of physical properties (browning, color, and firmness) as well as chemical properties including total phenol content (TPC), polyphenol oxidase (PPO) activity, malondialdehyde (MDA) concentration, respiration and ethylene production rates, sugar content and concentration, titratable acidity and vitamin C content.
Abiu fruit were obtained from farmers' gardens in West Java Province, Indonesia. Located at longitude 60.21°E, latitude 106.44°S, and 200 m above sea level. The fruit were harvested when fully ripe (65 days after fruit set) with bright yellow peel color. Harvest was performed in the morning, and fruit were brought to the laboratory within 30 minutes. On arrival, surface dirt was removed by dipping into water then dried by air blower. Fruit were then selected for color, shape, absence physical defects, and weight (250 ± 25 g) to ensure uniform characteristics. Fruit were then separated into treatment groups of 25 fruit in triplicate (75 fruits total per treatment). Each treatment replicate was soaked in the respective treatment solution for 10 minutes. The treatments were: water (control), 5 mM ascorbic acid (AA5), and 10 mM ascorbic acid (AA10). After treatment, the fruit were air-dried and placed in a container (80 cm × 60 cm × 45 cm) without a lid, then stored in an ambient room (28 ± 1°C and 80 ± 5% RH) for 12 days. Fruit sampling was carried out on days 0, 3, 6, 9, and 12 (storage periods).
Measurements of weight loss and fruit firmnessFruit weight loss was calculated using the method proposed by Kaewjumpol et al. (2021). The difference between fresh fruit weight before and after storage was calculated and expressed as a percentage of the initial fresh weight. The weight loss was expressed in a percentage (%).
The fruit firmness was measured using a Brookfield digital texture analyzer. The texture analyzer was set to mode 20, with a maximum load of 10 kg, a pressing depth of 15 mm, a speed of 60 mm·min−1, and a needle probe diameter of 2 mm. The firmness measurement of the abiu fruit was taken in triplicate at the equatorial section of the fruit. Firmness is expressed in Newton (N) (Arif et al., 2022).
Peel fruit color and browning index (BI) determinationColor changes were measured according to the method described by Chinwang et al. (2011). Peel fruit color was evaluated for lightness (L) and hue angle at 3 different positions using a Colorimeter CR-400 Chroma Meter (Konica Minolta Inc., Tokyo, Japan). Then, the average was determined.
The BI was evaluated by the method described by Arif et al. (2022). Browning was assessed by measuring the percentage of browning area on fruit peel and pulp, then assigned a score using the following scale: 0 = no browning; 1 = < 25% browning; 2 = 25–50% browning; 3 = 50–75% browning; 4 = > 75% browning. The BI was calculated using the following equation: BI = [Σ (browning scale) × (number of fruits in each scale)/(total number of fruits × the highest scale number)] × 100. The BI was expressed in a percentage (%). The non-marketability of fruit was indicated by a peel browning index ≥ 40% (Lichanporn et al., 2020).
Measurements of total phenolic content (TPC), Polyphenol Oxidase (PPO), and Malondialdehyde (MDA)The TPC in fruit pulp was analyzed by the Folin-Ciocalteu method (Chimvaree et al., 2020). Whereby 150 μL of supernatant, 2,400 μL of distilled water, and 150 μL of 0.25 N Folin-Ciocalteu were thoroughly mixed in a test tube by vortex. The mixture was allowed to react at room temperature for 3 min, and 300 μL of 1 N Na2CO3 solution was added. The solution was well mixed and incubated at room temperature (25°C) for 2 h. Then, a 200 mL sample, standard or blank from the assay tube was transferred to a clear 96-well microplate and the absorbance at 765 nm recorded for each well. TPC was then evaluated based on absorbance at 765 nm. The standard gallic acid (GAE) curve was plotted, with TPC concentration expressed as mg GAE·kg−1 fresh weight.
The PPO activity was measured according to the method described by Jiang (1999) with some modifications. The sample (1 g) was homogenized in 4 mL 0.1 M sodium phosphate buffer (at pH 7.2) and centrifuged at 12,000 × g for 30 min at 4°C. The supernatant was collected as an enzyme extraction solution. Then 100 mmol·L−1 citrate buffer (1.45 mL, pH 6.8) was added to 50 μL of the crude enzyme extract and 0.50 mL of 4-methyl catechol solution (100 mmol·L−1). One PPO activity unit (U) was defined as the quantity of enzyme that increased the absorbance at 420 nm by 0.001 per minute. PPO activity is expressed as U·g−1 protein.
The MDA concentrations were measured using the method of Xi et al. (2017). The sample (1 g) was homogenized in 5.0 mL of 5% (w/v) trichloroacetic acid (TCA) and centrifuged for 20 min at 10,000 × g. Next, the supernatant was collected and mixed with 0.67% TBA in a 1:1 (v/v) ratio, heated at 100°C for 20 minutes, and immediately cooled on ice. Then centrifuged at 10,000 × g for 10 min, with the absorbance of the supernatant at 450, 532, and 600 nm measure by spectrophotometer (UV-2600; Shimadzu Co., Kyoto, Japan). Finally, the MDA concentration was calculated using the formula: 6.45 × (A532 − A600) − 0.56 × A450. The MDA concentration was expressed as μmol·kg−1 based on fresh weight.
Respiration, ACC, and ethylene productionThe respiration rate and ethylene production during storage were measured using a Gas Analyzer (Type F-950; Felix instruments, Camas, WA, USA) by flowing air in a sealed glass bottle into the Gas Analyzer with an airflow rate of 70 mL·min−1. The respiration rate is expressed as mL·kg−1·h−1, and ethylene production is expressed as μL·kg−1·h−1 (Arif et al., 2022).
The ACC quantification was measured according to Lizada and Yang (1979) method. The GC Merck Varian 450 was used with a detector temperature of 100°C and a column temperature of 80°C with a gas flow rate of N2 0.6 mL·min−1. The ACC quantification is expressed as mL·kg−1.
Soluble sugar concentration and content (sucrose, glucose, and fructose)The soluble sugar concentration (SSC) of the fruit juice was determined using a digital Atago PR-1 refractometer (Atago Co. Ltd., Tokyo, Japan) at 28 ± 1°C according to the method of Asiche et al. (2017) and expressed as Brix (°Brix).
The sugar content (glucose, sucrose, and fructose) was measured following the method reported by Zhou et al. (2020). Aquabidest was added abiu pulp (5 g) until the volume reached 20 mL, ultrasonically agitated for 30 min, then passed through a microfilter. Supernatants were analyzed using ultra-high performance liquid chromatography (UHPLC) Merk Dionex Ultimate 3000 RS Column Compartment (mobile phase: acetonitrile, column: C18, flow rate: 1 mL·min−1, detector: change aerosol detector, and temperature: 25°C). The sucrose, glucose, and fructose contents are expressed as percentages.
Titratable acidity and vitamin CThe NaOH titration method described by Asiche et al. (2017) was used to determine the titratable acidity (TA) content. TA was determined by titrating the extract against 0.1 N NaOH and then expressed as percentage citric acid equivalents.
Vitamin C content was evaluated using the method of Klimczak and Swiglo (2015). Abiu pulp (1 g) was placed into a scaled test tube, with 0.0065 N H2SO4 added until the volume reached 20 mL, ultrasonically agitated for 30 minutes, then filtered through a 0.22 μm filter membrane. Vitamin C content was analyzed by UHPLC Merk Dionex Ultimate 3000 RS Column Compartment (mobile phase: H2SO4; column: variant C18, 150 mm long, 4.6 mm in diameter, and 5 μm particle size; flow rate: 1 mL·min−1, detector: diode array detector; wavelength: 254 nm; and temperature: 25°C). Vitamin C was expressed as mg·10−2·g−1.
Statistical analysisExperiments were performed using a randomized complete block design with three replications. Means were compared by one-way analysis of variance and Duncan multiple range test (DMRT) at a 5% significance level. Data are presented as mean ± standard error (SE).
Weight loss is critical in determining fruit’s potential shelf life (Ribeiro and de Freitas, 2020). Weight loss may occur due to increased metabolic activity and/or decreased membrane integrity caused by increased postharvest senescence (Koyuncu et al., 2019). This current study observed continual weight loss in abiu fruit during storage, but weight loss for abiu fruit with the AA10 treatment was less than the control (Fig. 1). Weight loss in the abiu fruit on day 12 of storage reached 24.25, 24.12, and 17.74% for the control, AA5 treated, and AA10 treated fruit, respectively (Fig. 1). This demonstrated that the AA10 treatment reduced fruit weight loss by 6.51%, a 27% improvement on the control. Exogenous application of ascorbic acid to lychee fruit inhibited postharvest senescence and weight loss (Ali et al., 2021) and in strawberries, reduced water loss and weight loss during storage (Sogvar et al., 2016). Thus, a 10 mM ascorbic acid treatment will likely reduce metabolic activity, delay senescence, and maintain cellular integrity, thereby reducing weight loss in ambiently stored abiu fruit.
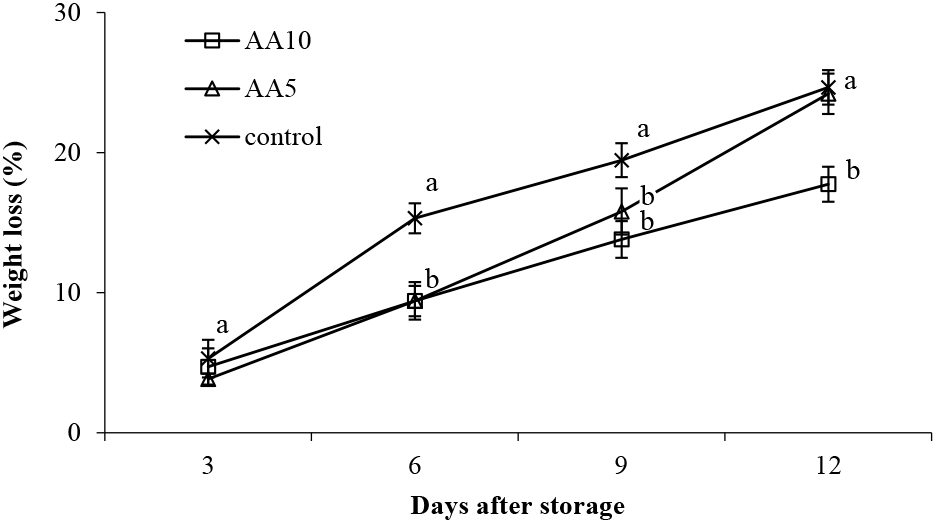
Effect of ascorbic acid treatments on abiu fruit weight loss during storage. Data are means of three replicates ± SE. Different letters in the same day indicate significant difference by the Duncan multiple range test (P < 0.05).
Fruit firmness is an important parameter consumers use in assessing fruit quality. The process of fruit ripening induces cell wall modifications, decreasing fruit firmness (Wang et al., 2018; Zhang et al., 2019). As shown in Figure 2, abiu fruit firmness decreased gradually for all treatments during storage. On day 12, the control fruit firmness was 8.05 N. However, the abiu fruit firmness with AA10 treatment was 9.87 N, 1.23-fold firmer than the control and 1.10-fold firmer than the AA5 treatment (Fig. 2). In this study, the higher concentration exogenous ascorbic acid treatment maintained abiu fruit firmness better during storage. In grapes, ascorbic acid is known to inhibit polygalacturonase (PG) enzyme activity, and through this, maintain grape firmness (Lo’ay and El-Boray, 2018). Additionally, ascorbic acid may maintain fruit firmness by reducing membrane lipid peroxidation, enhancing cell capacity to scavenge ROS, and decreasing fruit respiration (Liu et al., 2014).
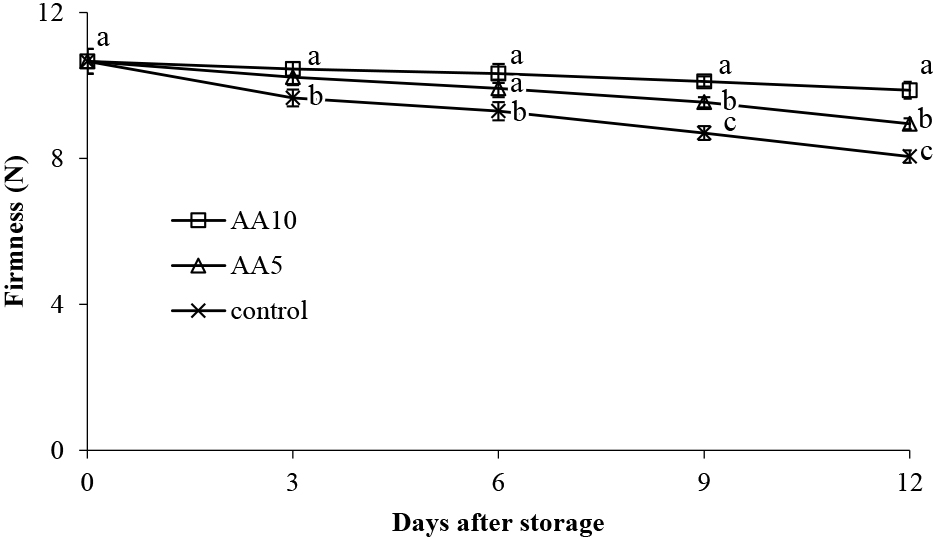
Effect of ascorbic acid treatments on abiu fruit firmness during storage. Data are means of three replicates ± SE. Different letters in the same day indicate significant difference by the Duncan multiple range test (P < 0.05).
Color is an essential external indicator of fruit quality. Hue and lightness values in abiu peel decreased for all treatments during storage (Fig. 3). The average hue and lightness of the abiu peel at harvest were 87.99 and 72.71, respectively. During storage, the AA10 treatment inhibited the decrease in hue and lightness values of abiu fruit. At the end of storage, the hue value of the AA10 and AA5-treated fruit were higher than the control (Fig. 3a). This behavior may be due to ascorbic acid delaying fruit color development (Martinez-Ortiz et al., 2017). In addition, ascorbic acid can also suppress ethylene production. This is important as ethylene is essential in fruit pigmentation to regulate the expression of enzymes that influence fruit color (Montalvo et al., 2009). Abiu peel exhibited similar changes, with day 12 lightness decreased compared to fruit at day 0 of storage by 19.02, 7.64, and 2.85 units, for control, AA5-, and AA10-treated fruit, respectively (Fig. 3b). The L value in abiu fruit also relates to the progress of browning during storage. The L value correlated negatively with litchi browning index, with decreased L values indicated a higher browning percentage (Zhang et al., 2015). Discoloration during browning primarily relates to tissue darkening (Peng et al., 2021). Thus, the AA10 treatment of abiu fruit delays decreases in hue and L values during storage.

Effect of ascorbic acid treatments on color; a) hue, and b) lightness of abiu fruit during storage. Data are means of three replicates ± SE. Different letters in the same day indicate significant difference by the Duncan multiple range test (P < 0.05).
Browning is a primary factor dictating shelf life and fruit quality during storage (Li et al., 2021; Wang et al., 2021). Abiu fruit peel and pulp browning gradually increased during storage (Fig. 4). An important consequence of browning is the negative impact on consumer preference. In apples, severe browning (> 30% relative area) is reported to result in 50% of consumers discarding the whole apple (Jaeger et al., 2018). Lychee fruit also loses marketability when surface browning exceeds a score of 3.0 (Ali et al., 2021). Browning of the abiu peel increased gradually during storage for all treatments (Fig. 5a). However, peel browning in the control was higher than for ascorbic acid-treated fruit during storage. Overall, compared to the control, the ascorbic acid treatment inhibited abiu peel browning by 6.25-fold and 1.88-fold for the AA10 and AA5 treatments, respectively. The BI in abiu peel, on days 3 and 6 of storage was > 10% for both the control and AA5 treatment, while the AA10 treatment BI remained < 8% until day 12 of storage.

The browning symptom in abiu fruit during storage. AA5 = ascorbic acid 5 mM, and AA10 = ascorbic acid 10 mM.

Effect of ascorbic acid treatments on; a) peel browning index, and b) pulp browning index of abiu fruit during storage. Data are means of three replicates ± SE. Different letters in the same day indicate significant difference by the Duncan multiple range test (P < 0.05).
In addition, the pulp browning index of the control was > 50% by day 12 of storage (Fig. 5b). On day 12, pulp browning of the AA10- and AA5-treated fruit was inhibited by 5-fold and 1.47-fold, respectively, compared with the control. For the control and AA5-treated fruit, a pulp BI > 30% occurred by day 3 and day 6, respectively. For the AA10-treated fruit, the pulp BI remained < 11% until day 12 of storage. Therefore, the control abiu fruit lost market value after three days of storage, while the AA10-treated abiu fruit retained marketable value until day 12 of storage. Thus, the AA10 treatment effectively extends shelf-life and given the simple treatment process, appears appropriate for use in the abiu fruit industry.
As an antioxidant, ascorbic acid promotes o-quinone regeneration and prevents polymerization into brown pigments (Franck et al., 2007; Moon et al., 2020). Antioxidants react with oxygen, thus inhibiting the initiation of browning. Despite ascorbic acid not directly interacting with PPO enzymes, it inhibits enzymatic browning by chemically reducing oxidized substrates. This chemical reduction process of ascorbic acid is reported to be reduction of the enzymatically formed o-quinone to di-phenol (Moon et al., 2020).
TPC, PPO, and MDAEnzymatic browning is caused by three main factors: phenolic compounds, polyphenol oxidases, and oxygen (Moon et al., 2020). Phenolic compounds are substrates for the PPO enzymes involved in the browning reaction (Zhang et al., 2022). TPC in abiu fruit decreased during storage for all treatments (Fig. 6). At harvest, the TPC average was 99.85 mg·10−2·g−1; on day 12 of storage, the TPC of abiu fruit in the control dropped 7.68-fold. However, the AA10 treatment inhibited phenolic degradation significantly more than the control and AA5 treatment. On average, AA10-treated abiu fruit exhibited 2.11-fold higher concentrations of TPC than the control and 1.38-fold higher than the AA5 treatment on day 12 of storage. Phenolics after acid treatment were higher than controls in lychee fruit (Ali et al., 2019; Kumari et al., 2015), mango (Lo’ay and Ameer, 2019), and guava (Lo’ay and Taher, 2018). The phenolic content of all abiu fruit decreased, associated with increased enzymatic activity. However, this was more pronounced for the control fruit than for treated fruit, as more damaged cells lead to increased enzyme activity and increased browning. Phenolic compounds are PPO enzyme substrates, involved in fruit browning reactions. As an antioxidant, ascorbic acid inhibits phenol degradation and the conversion of phenol to quinone (Moon et al., 2020). Ascorbic acid also inhibits the browning reaction by reducing o-quinones back to the phenolic substrates (Soliva-Fortuny and Martín-Belloso, 2003). Thus, the AA10 treatment for abiu fruit suppresses phenolic compound oxidation, which inhibits further browning.

Effect of ascorbic acid treatments on abiu fruit total phenolic content during storage. Data are means of three replicates ± SE. Different letters in the same day indicate significant difference by the Duncan multiple range test (P < 0.05).
Besides phenolic compounds, PPO enzyme activity is another factor in browning (Liu et al., 2022). PPO enzyme activity was observed to increase for all treatments during storage (Fig. 7). PPO enzyme activity was observed to increase 6-, 5-, and 5-fold during storage for the control, AA5, and AA10 treatments, respectively. PPO enzyme activity is optimal at pH 5–7, with a lower pH inhibiting enzymatic activity (Queiroz et al., 2008). As a weak acid, ascorbic acid decreases pH, inhibiting PPO enzyme activity. However, PPO enzyme activity in ascorbic acid treated abiu fruit increased during storage. This indicates that the ascorbic acid does not react directly with the enzyme in suppressing PPO enzyme activity. Indeed, Arias et al. (2007) reported, in pears, that rather than direct PPO enzyme activity inhibition by ascorbic acid, it acts on the oxidized substrate molecules.
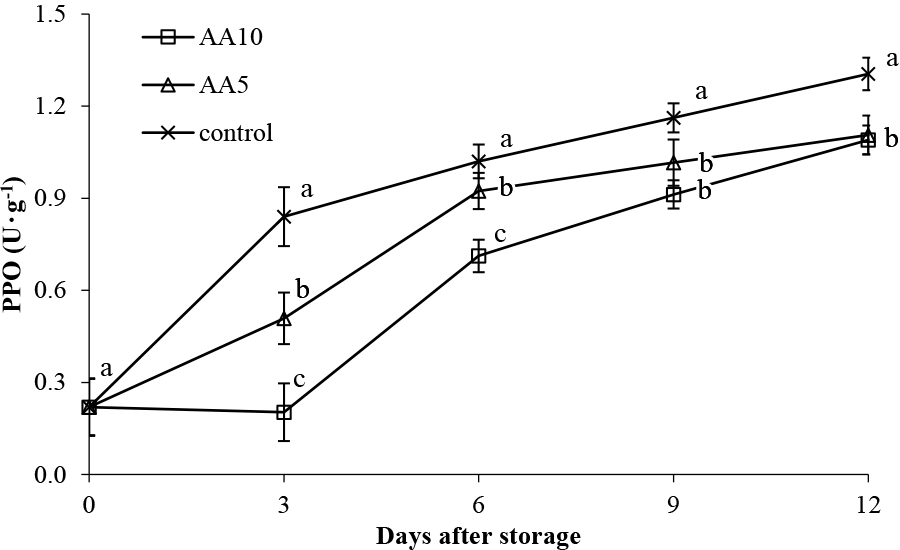
Effect of ascorbic acid treatments on abiu fruit PPO during storage. Data are means of three replicates ± SE. Different letters in the same day indicate significant difference by the Duncan multiple range test (P < 0.05).
MDA is a product of membrane lipid peroxidation and is a useful indicator for assessing oxidative damage to plant tissues (Saleem et al., 2021). The MDA concentration in abiu pulp increased linearly from day 0 to day 12 (Fig. 8). The AA10 treatment of abiu fruit reduced MDA production more than other treatments. The MDA concentration of exogenously AA10-treated Abiu fruit was 1.41-fold lower than the control (Fig. 8). As an antioxidant, ascorbic acid prevents reactive oxygen species (ROS) formation and reduces oxidative stress in tissues (Ali et al., 2021; Saleem et al., 2021). One ROS product is MDA, with MDA accumulation damaging cell membranes composition, promoting brown polymer formation, and increasing browning (Lin et al., 2016). Increased MDA content damages cellular membrane structural integrity, promoting contact between PPO enzymes and phenolic substrates, leading to additional browning (Lin et al., 2014). Therefore, the AA10 treatment of abiu fruit may significantly inhibit increases in MDA content during storage.
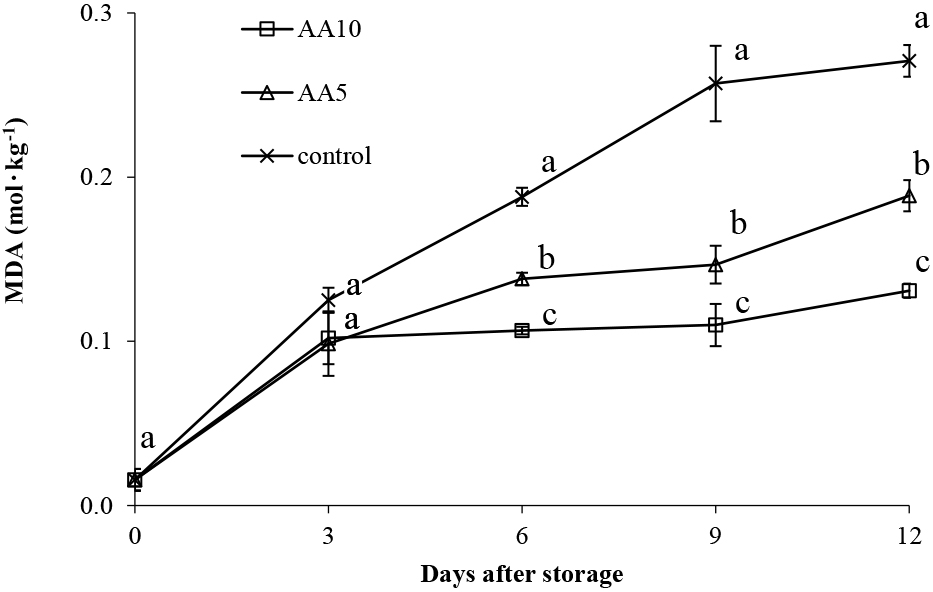
Effect of ascorbic acid treatments on abiu fruit MDA during storage. Data are means of three replicates ± SE. Different letters in the same day indicate significant difference by the Duncan multiple range test (P < 0.05).
Ascorbic acid treatment affected respiration rate, ACC concentration, and ethylene production (Fig. 9). The CO2 production of abiu fruit decreased over the first three days of storage then increased until the climacteric peak was reached for each treatment (Fig. 9a). The peak respiration rate (CO2 production and O2 consumption) occurred for the control fruit on day 5 (Fig. 9a, b), while, for the AA10-treated fruit, the peak respiration rate occurred on day 8. Ascorbic acid treatment is previously reported to suppress the respiration rate in guava (Martinez-Ortiz et al., 2019). In addition, the O2 concentration of AA10-treated abiu fruit was relatively constant (stable) over the storage period, which may reflect reduced deterioration and senescence. Ayon-Reyna et al. (2019) reported ascorbic acid induced O2 consumption stabilization, inhibited pineapple deterioration, and slowed senescence. From these results, the AA10 treatment delays abiu fruit respiration rate, thus further extends shelf life.

Effect of ascorbic acid treatments on respiration; a) CO2 production, and b) O2 consumption of abiu fruit during storage. Data are means of three replicates ± SE. Different letters in the same day indicate significant difference by the Duncan multiple range test (P < 0.05).
The ACC concentration in AA10-treated fruit tended to be less than in the other fruit (Fig. 10a). The highest concentration of ACC in abiu fruit occurred on day 6 for the control and AA5 treatment. In contrast, the ACC maximum concentration for the AA10-treated fruit occurred on day 9. The fruit senescence inhibiting effect of ascorbic acid is related to inhibition of ACC production, ethylene, and fruit respiration (Martinez-Ortiz et al., 2019). ACC production in guava coated with ascorbic acid is inhibited during storage (Martinez-Ortiz et al., 2019). ACC synthase is a key ethylene biosynthesis regulatory enzyme, catalyzing ethylene precursor production from S-Adenosyl-Methionine (SAM). Furthermore, peak ethylene production in the control abiu fruit occurred on day 5 (Fig. 10b). Meanwhile, peak ethylene production for the AA5- and AA10-treated fruit were 2 and 3 days later than the control, respectively. Thus, the respiration rate was slowed, and ethylene production decreased using the AA10 treatment. This further supports the AA10 treatment as extending abiu fruit shelf life.

Effect of ascorbic acid treatments on; a) ACC, and b) ethylene production of abiu fruit during storage. Data are means of three replicates ± SE. Different letters in the same day indicate significant difference by the Duncan multiple range test (P < 0.05).
The SSC is an important indicator determining fruit quality and consumer acceptance (Crisosto and Crisosto, 2005). The SSC values of the control abiu fruit gradually increased from day 0 until day 6 of storage, then steadily decreased until day 12 of storage (Fig. 11). The SSC increase in early storage is associated with the degradation of carbohydrate polymers into their sub-units. After 6 days of storage, the SSC decreased due to the high metabolism in the fruit pulp and the processes leading to senescence. The SSC of abiu fruit on day 0 was 12.54 °Brix, this value increased 2.19, 1.68, and 1.03 °Brix by day 12 of storage for the control, AA5-, and AA10-treated fruit, respectively (Fig. 11). For the AA10-treated fruit, exogenous ascorbic acid inhibited SSC increase, reducing the SSC by 1°Brix at the end of storage, compared to the control. The AA10 treatment efficiently delayed starch and organic acid conversion to sugars. This behavior was also observed in strawberries with ascorbic acid treatment suppressing SSC increase by ≤ 1 °Brix compared to control strawberries (Saleem et al., 2021).
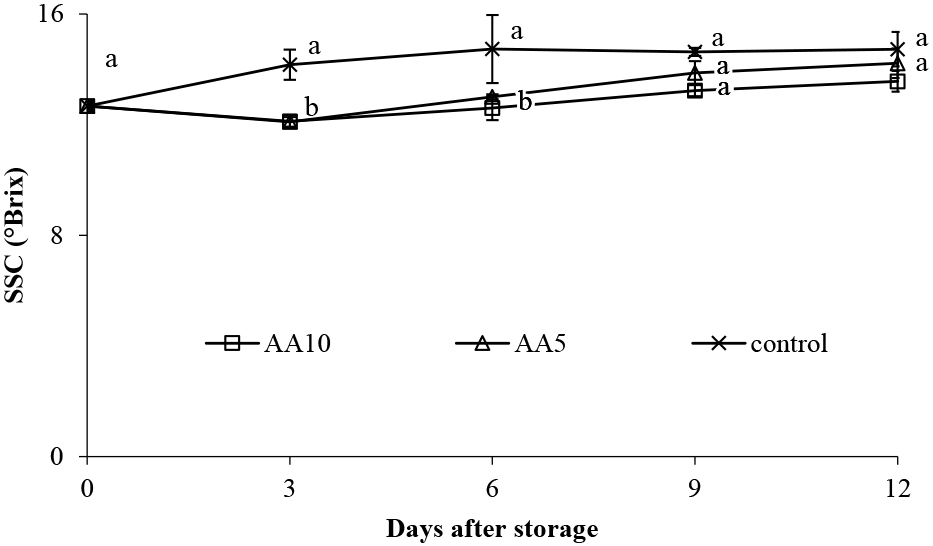
Effect of ascorbic acid treatments on abiu fruit SSC during storage. Data are means of three replicates ± SE. Different letters in the same day indicate significant difference by the Duncan multiple range test (P < 0.05).
The sucrose content in abiu fruit increased until the climacteric peak, then decreased until the end of the storage (Fig. 12a). This sucrose content increase is thought to be due to starch degradation to sucrose until the climacteric peak occurs. Starch content continues to decrease until the climacteric peak, after which, sucrose is hydrolyzed faster into glucose and fructose than it is formed (Agopian et al., 2011). Exogenous ascorbic acid treatment maintains higher sucrose content and slows the conversion of sucrose into reducing sugars (glucose and fructose) which stabilizes longan fruit taste and nutritional attributes during storage (Liu et al., 2021). Exogenous ascorbic acid treatment also suppresses polygalacturonase (PG) activity, which is responsible for polysaccharide hydrolysis, including sucrose (Lo’ay and El-Boray, 2018).
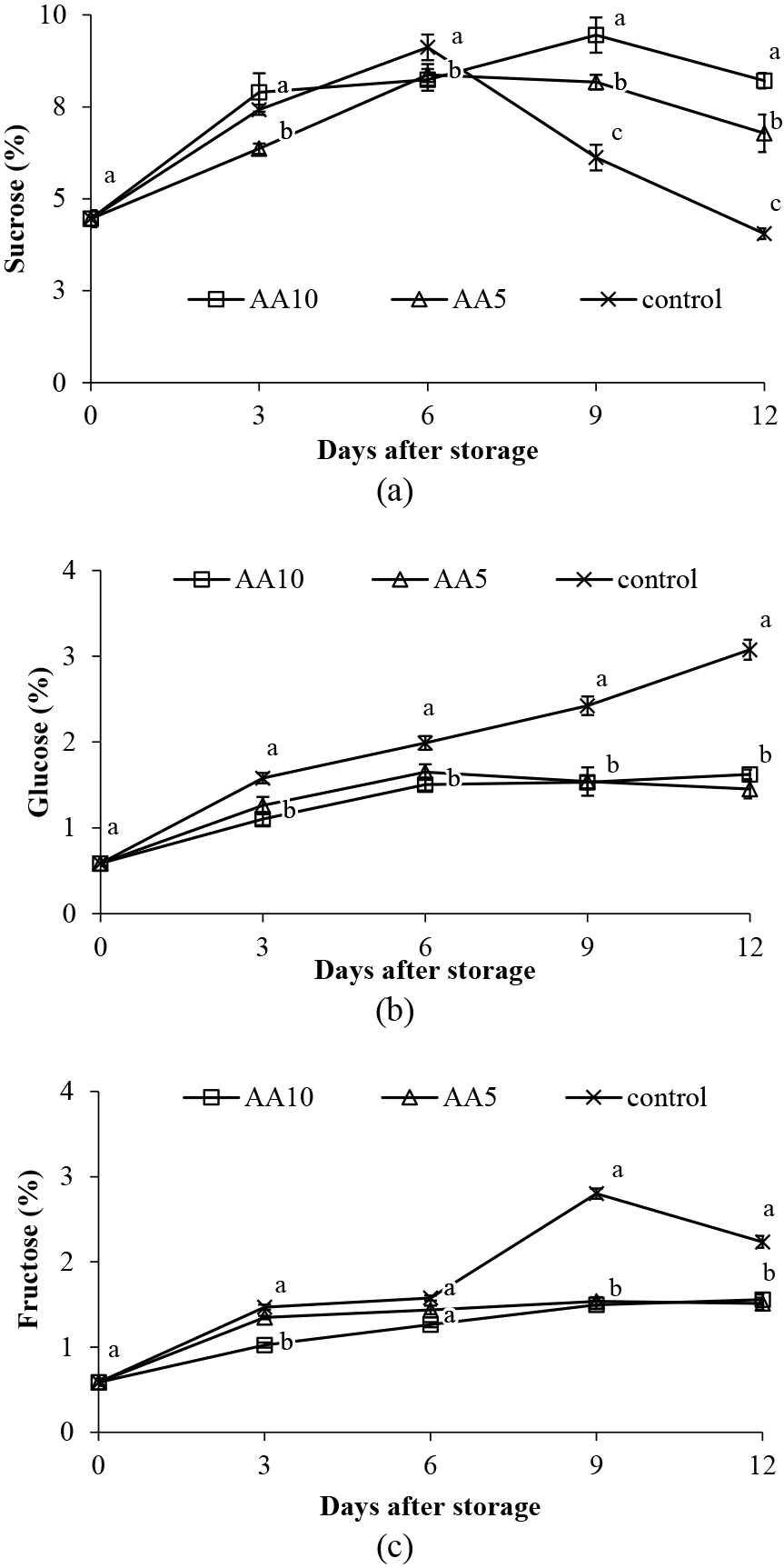
Effect of ascorbic acid treatments on; a) sucrose, b) glucose, and c) fructose levels in abiu fruit during storage. Data are means of three replicates ± SE. Different letters in the same day indicate significant difference by the Duncan multiple range test (P < 0.05).
Fruit glucose and fructose content increased during storage, but the glucose and fructose levels for ascorbic acid treated fruit were lower than the control (Fig. 12b, c). We suspect that it is caused by the reduced rate of sucrose hydrolysis and starch breakdown. Tao et al. (2021) reported that organic acid treatment inhibited increases in glucose and fructose content for tomato fruit during storage, where the acid treatment increased sucrose phosphate synthase (SPS) enzyme activity and decreased acid invertase (AI) enzyme activity.
TA and vitamin CTA and vitamin C are also important markers of fruit quality. As shown in Figure 13, TA in abiu fruit decreased over storage for all treatments. However, AA10 treatment substantially inhibited TA loss during storage. TA in the AA10-treated fruit was 3.57-fold higher than the control and 1.23-fold higher than the AA5 treatment on day 12 of storage. During respiration, organic acids are converted to sugars, and their derivatives or use during storage may be a contributing factor to the TA decrease (Tilahun et al., 2019, 2020). The exogenous ascorbic acid treatment of abiu fruit potentially delays the consumption of organic acids inherent in the fruit, which inhibits changes in TA. Thus, the AA10 treatment of abiu fruit maintains the TA content during the storage period.
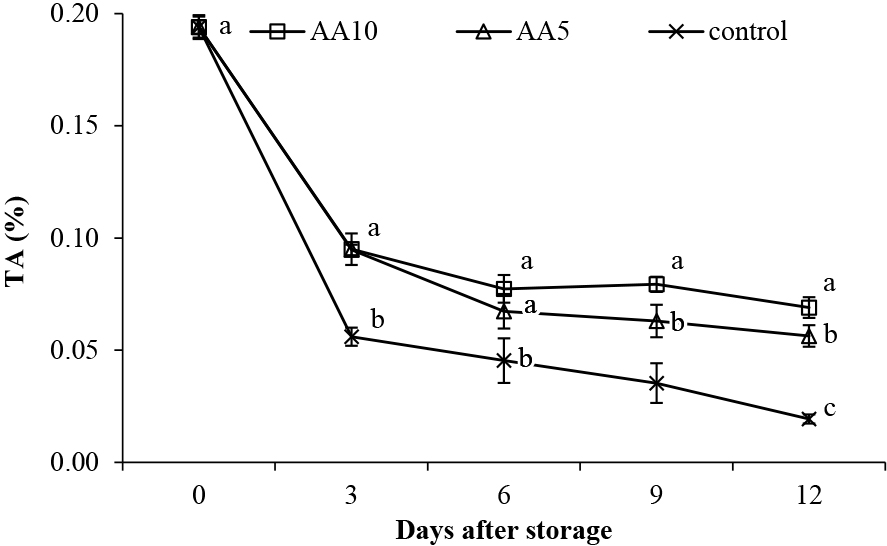
Effect of ascorbic acid treatments on abiu fruit TA during storage. Data are means of three replicates ± SE. Different letters in the same day indicate significant difference by the Duncan multiple range test (P < 0.05).
Vitamin C is an essential nutrient and, during storage, is more susceptible to oxidation compared to other nutrients (Veltman et al., 2000). Vitamin C levels in abiu fruit decreased during the storage period for all treatments (Fig. 14). However, the AA10 treatment significantly inhibited vitamin C decreases compared to the control and AA5 treatment. On day 12 of storage, the fruit vitamin C content was 16.15 and 16.33 mg·10−2·g−1 for the control and AA5 treatment, respectively. Meanwhile, for AA10-treated abiu fruit, the vitamin C content was 1.44-fold higher than the control. The AA10 treatment of abiu fruit preserved vitamin C content during storage. Thus, the exogenous ascorbic acid treatment maintains vitamin C content, retaining fruit antioxidant properties. Arafat (2009) also reported that, for mangoes, exogenous ascorbic acid treatment retained vitamin C content and functioned as an antioxidant during the storage period. Vitamin C is an important antioxidant that can delay senescence in fruit and reduce reactive oxygen species (Razzaq et al., 2015).

Effect of ascorbic acid treatments on abiu fruit vitamin C levels during storage. Data are means of three replicates ± SE. Different letters in the same day indicate significant difference by the Duncan multiple range test (P < 0.05).
In conclusion, our study demonstrated an exogenous ascorbic acid treatment at a concentration of 10 mM increased abiu fruit shelf life up to 12 days, nine days longer than the control. In addition, the AA10 treatment of abiu fruit; 1) inhibited weight loss, browning, and MDA, 2) reduced respiration rate, ethylene production, and phenolic oxidation, and 3) maintained vitamin C and fruit firmness. Therefore, the AA10 treatment is an excellent candidate as a treatment to extend abiu fruit shelf life and maintain quality.
The author would like to thank financial support for this research provided by Directorate General of Higher Education, Research and Technology, Ministry of Education, Culture, Research and Technology, Republic of Indonesia in accordance with The Research Implementation Assignment Agreement for the fiscal year 2022 No.: 001/E5/PG.02.00PT/2022 and Letter of Assignment No.: 3665/IT3.L1/PT.01.03/P/B/2022.