2023 年 92 巻 3 号 p. 207-215
2023 年 92 巻 3 号 p. 207-215
The effect of short-term anoxic treatment prior to storage at ambient and cool temperatures on pericarp browning, fruit quality, secondary metabolites, antioxidant activity, and the browning enzyme of litchi (Litchi chinensis Sonn.) cv. Hong Huey were investigated. Litchi fruit were exposed to anoxic conditions for 6, 12, 18 and 24 h before storage at 28 ± 2°C for 5 days, or at 7 ± 2°C for 14 days. Anoxic treatment resulted in significantly decreases in electrical conductivity, weight loss, browning index, while maintaining the total soluble solids (TSS) and delaying increases in polyphenol oxidase (PPO) and peroxidase (POD) activities compared with control fruit. Furthermore, anoxic treatment increased litchi pericarp methanol extract antioxidant capacity, as measured by free-radical scavenging activity. This is associated with greater amounts of ascorbic acid, anthocyanins, and phenolic/flavonoid components as compared with control fruit. Additionally, anoxic treatment considerably delayed litchi fruit pericarp browning. This suggests that with adequate short-term anoxia duration, an enhanced non-enzymatic antioxidant process may directly or indirectly delay litchi pericarp browning. Thus, a short anoxic treatment enables harvested litchi quality to be sustained at ambient and cool temperatures. This non-chemical and inexpensive treatment deserves further development and application, especially in commercial distribution systems where cooling is insufficient.
Litchi, or lychee (Litchi chinensis Sonn.) is a subtropical fruit with high commercial value due to its slightly acid taste, semi-translucent white aril, excellent aroma, and attractive red skin (Holcroft and Mitcham, 1996). Litchis originated in southern China and are now commercially grown in Thailand, the world’s fourth largest producer of litchi (Yapwattanaphun and Subhadrabandhu, 2001). However, litchi production is constrained by its short shelf life, resulting in a rapid skin color change from red to brown and fruit perishability. Postharvest treatments are used to delay browning, extend shelf life, and improve various litchi qualities during transportation and storage. Many approaches, such as coating, acid dipping, hot water treatment, and controlled atmosphere storage, are used to prevent litchi fruit pericarp browning (Sivakumar et al., 2010). For delaying litchi skin’s red color loss, postharvest sulfur fumigation is most effective. However, due to food safety and chemical use concerns, alternative methods to control pericarp browning and extend litchi fruit shelf life are required.
Pericarp browning of litchi fruit is primarily attributed to the degradation of red anthocyanin pigments in association with enzymatic oxidation of phenolics by polyphenol oxidase (PPO) and/or peroxidase (POD) (Jiang et al., 2004a; Zhang et al., 2000). As an aerobic anthocyanase-anthocyanin-phenolic-PPO reaction may be involved in litchi enzymatic browning (Zhang et al., 2000), lowering O2 levels may decrease the enzyme activity responsible for litchi pericarp oxidation. Benefits of short-term anoxic conditioning storage of fruit and vegetables include decreased respiration rate, delayed broccoli floret yellowing (Techavuthiporn et al., 2021), and lower incidences of physiological disorders (Benkeblia, 2021). The beneficial effects of anoxia varied according to fruit type. Anaerobic metabolites can be beneficial in small amounts, but, in large quantities, may also be harmful, potentially spoiling the essential produce qualities of odor and flavor. For litchi fruit, pericarp tissue membrane integrity appears correlated to anoxic treatment (Liu et al., 2007). Litchi exposure to six hours of anoxic conditions considerably delayed skin browning, inhibited disease development, and maintained fruit quality (Jiang et al., 2004b). Increasing treatment exposure duration may further influence pericarp browning and litchi shelf life, either positively or negatively. Plants exhibit various response and tolerances based on their species’ structural and metabolic adaptations. This response directs molecular mechanism’s behavior in the absence of oxygen (Benkeblia, 2021). Furthermore, some plants are exceptionally resistant to anoxia.
Litchi ‘Hong Huey’ is an important commercial cultivar in Northern Thailand (Yapwattanaphun and Subhadrabandhu, 2001). ‘Hong Huey’ is egg- to spherical-shaped, with sharp-pointed protuberances, and a rough-surfaced pericarp containing high levels of flavonol and A-type-linked procyanidin (Reichel et al., 2013). There are few studies on the effect of short-term anoxic treatment duration on litchi fruit quality. The objective of this study is to determine optimal anoxic treatment conditions for postharvest litchi handling and storage by examining the influence of pre-storage anoxic treatment length on antioxidant activity, pericarp browning enzymes, and litchi fruit physicochemical characteristics during ambient and cool storage.
Fresh litchi fruit (Litchi chinensis Sonn.) cv. Hong Huey at a commercially mature stage were harvested from an orchard located in the Maejai district, Phayao province, Thailand. Fruit were delivered to the laboratory in a temperature-controlled vehicle within 1 h. Before treatment, litchi fruit were selected with complete brilliant red skin, consistent size, and no defects or infections.
Fruit were randomly divided into five groups (100 fruit per group) with three replications. Fruit from each group were placed in 10 L plastic containers and flushed with humidified ultrapure N2 (99.999%) at a flow rate of 100 mL·min−1. The flow was maintained until the container O2 concentration was less than 0.05% (v/v) using a 6600 headspace O2 and CO2 analyzer (Illinois Instruments Inc., Illinois, IL, USA). The fruit were maintained for 6, 12, 18, and 24 h under anoxic conditions. The control group was flushed with humidified ambient air at the same flow rate used for the N2 gas. After removal from the containers, fruit samples were placed in plastic baskets and covered with polyethylene (PE) sheets to maintain humidity (85–95%), then stored at an ambient temperature (28 ± 2°C) for up to 5 days, or at a cool temperature (7 ± 2°C) for 14 days. All samples were lyophilized and stored at −20°C until determination of bioactive components and enzymatic browning activity.
Each treatment was randomly applied to three replicates (15 fruit per replicate). Fruit that did not undergo N2 treatment served as the 0-day sample.
Pericarp browning index and color measurementThe fruit peel was assessed using five ranks of browning stages according to the pericarp browning area using the following scale: browning stage 0, no browning; 1, ≤ ¼ browning; 2, ¼ – ½ browning; 3, ½ – ¾ browning; 4, ¾ – complete browning. The browning index of the litchi pericarp was calculated from the browning scale and the percentage of corresponding fruit within the class.
Litchi color was measured at the fruit surface and recorded as lightness (L*) and redness (a*) in the Hunter L*a*b* color system using a CR-400 Minolta chroma meter (Konica Minolta Co., Tokyo, Japan).
Weight loss (%) and electrical conductivityLitchi fruit weight was regularly measured by digital balance. Weight loss was computed as a percentage of the sample’s starting weight at 0 d and at the end of each storage interval. Pericarp discs of 2 g (30 discs) were obtained using a cork borer (10 mm in diameter) and were incubated in 25 mL of distilled water for 30 min to test electrical conductivity. Afterwards, the solutions conductivity was measured using an SC 2300 conductivity meter (Suntex Instruments Co., Ltd., New Taipei, Taiwan) at 25°C and expressed as mS·cm−1.
Total soluble solids (TSS), titratable acidity (TA), ascorbic acid content and pH of litchi pulpLitchi juice was obtained by squeezing litchi pulp and then filtered through three layers of cotton gauze. The TSS and pH of the juice were measured with a PAL-1 refractometer (Atago Co., Tokyo, Japan) and a PH-230SD pH meter (Lutron Electronic Enterprise Co., Ltd., Taipei, Taiwan), respectively.
The TA content was determined by titrating 5 mL of juice to pH 8.1 with 0.1 N NaOH and expressed as % malic acid.
Ascorbic acid content was assayed by the titrimetric method. One mL of juice was mixed with 5 mL of metaphosphoric acid-acetic acid solution before titration with dichlorophenolindophenol (dye solution).
Total phenolic, flavonoid, and anthocyanin contents of litchi pericarpTotal phenolic and flavonoid contents were analyzed according to the method of Jarerat et al. (2022) with slight modifications. One gram of lyophilized pericarp was homogenized in 80% ethanol and centrifuged at 12,000 × g for 20 min at 4°C. The supernatant was used in evaluating bioactive compounds and antioxidant activity. The reaction was initiated by combining the supernatant with Folin-Ciocalteu phenol solution. The mixture was then incubated at room temperature for 60 min with 2 mL of saturated Na2CO3 solution. The total phenolic content in the mixture was determined using an Evolution 201 UV-Vis spectrophotometer at 760 nm (Thermo Scientific, Madison, WI, USA) and expressed as milligrams of gallic acid equivalent per g dry weight (mg GAE·g−1).
To evaluate total flavonoid content, a solution containing 0.5 mL of supernatant, 0.5% NaNO2, 10% AlCl3·6H2O and 1 M NaOH was made. Total flavonoid content was estimated by measuring the absorbance at 510 nm and expressed as milligrams of catechin equivalent per g dry weight (mg CE·g−1).
Anthocyanin concentration of the extraction solution was determined according to the method of Zheng and Tian (2006). Pericarp tissue (1 g) was homogenized and extracted with 15 mL of HCl-methanol (0.15% HCl:95% methanol = 15:85) over 4 h. The extract was centrifuged at 10,000 × g for 20 min and the supernatant absorbance measured at 530, 620, and 650 nm by UV-Vis spectrophotometer. Anthocyanin content was then estimated using the following formula:
ΔA·mL−1 = (A530 − A620) − 0.1(A650 − A620).
The anthocyanin content was expressed as ΔA·g−1 fresh weight.
Antioxidant propertiesAntioxidant activity was determined using the DPPH (2,2-diphenyl-2-picrylhydrazyl) radical scavenging assay according to the method of Brand-Williams et al. (1995) with slight modification. The litchi pericarp extract was obtained as per that used for total phenolic measurement. The sample (150 μL) was added to 2,850 μL of DPPH in methanol. This was mixed then allowed to stand overnight at room temperature. The solution absorbance at 515 nm was measured by UV-Vis spectrophotometer. A standard curve was prepared using ascorbic acid and expressed as mg ascorbic acid·g−1 dry weight.
The Ferric Reducing Antioxidant Power (FRAP) assay was performed according to Jarerat et al. (2022) with some modifications. Briefly, a fresh working solution was prepared by mixing 25 mL of 300 mM acetate buffer (3.1 g C2H3NaO2·3H2O and 16 mL C2H4O2), pH 3.6, 2.5 mL of 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) solution, and 2.5 mL of 20 mM FeCl3·6H2O, and then warmed at 37°C. The litchi extract supernatant (150 μL) was added to 2,850 μL of the FRAP solution and allowed to react for 30 min in the dark. The absorbance at 593 nm of the colored ferrous-tripyridyltrizine complex was measured. The standard curve was linear between FeSO4 and FRAP solution and the results are expressed in μmol Fe (II)·g−1 dry weight.
Polyphenol oxidase (PPO) and peroxidase (POD) activitiesLitchi PPO and POD were extracted by homogenizing lyophilized pericarp (1 g) in 50 mM phosphate buffer at pH 6.5 and centrifuged at 12,000 × g at 4°C for 20 min. The crude enzyme extract was recovered from the supernatant and stored on ice until enzyme tests were performed. PPO activity was measured using the Arnnok et al. (2010) method, with 2.95 mL of 10 mM catechol as the substrate (freshly dissolved in phosphate buffer) and 0.05 mL of enzyme solution. The reaction was monitored at 410 nm for 2 min at room temperature using a UV-Vis spectrophotometer.
POD activity was assayed by the method of Arnnok et al. (2010) in which the reaction mixture (freshly prepared) contained 0.5 mL of 10 mM guaiacol solution (substrate), 1 mL of 10 mM H2O2 and 0.1 mL of enzyme solution. The reaction was monitored at 470 nm for 2 min at room temperature using a spectrophotometer. The blank sample contained the same mixture solution without the enzyme extract. Initial rates were calculated from the linear portion (initial velocity) of the reaction progress curve. One unit of enzymatic activity was defined as the amount of the enzyme that caused a change of 0.001 in absorbance per min. PPO and POD activities were expressed as unit·g−1 DW.
Statistical analysisExperiments were conducted using a completely randomized design (CRD). Analysis of variance (ANOVA) to evaluate treatment effects was performed using the SPSS software program version 18. Data is presented as the mean values of three replications ± SD.
The browning score showed an upward trend in all litchi fruit as storage time progressed (Fig. 1A). The pericarp browning score of anoxia-treated fruit was significantly (P < 0.05) lower than that of the control. The browning score of anoxia-treated fruit after 18 h was the lowest, followed by 6 h, 24 h, and 12 h after 5 days at 28°C. Conversely, the browning score of 12 h anoxia-treated litchi fruit increased after cool storage at 7°C for 14 days and was not significantly (P > 0.05) different from the control. As with browning score, anoxia influenced litchi pericarp lightness (L* value) and redness (a* value) retention (Fig. 1B, C). For control fruit, L* and a* values dramatically decreased, especially when stored at 28°C for 5 days. In contrast, fruit with 18 h anoxic treatment significantly (P < 0.05) maintained the highest values of L* and a*, indicating that exposure time to anoxia conditions retains litchi red color and delays pericarp browning. The visual characteristics of litchi fruit with anoxic treatments are also shown in Figure 2. Brown flecks on the control fruit pericarp were obviously noticeable compared to other samples.

Browning index (A), color L* value (B) and a* value (C) of litchi fruit with 6–24 h of anoxic treatment and stored at 28°C for 5 days or at 7°C for 14 days. Different lowercase or uppercase letters above bars indicate significant (P < 0.05) differences among anoxic treatments and storage periods, respectively.

Effect of anoxic treatment (h) on the visual appearance of litchi fruit stored at 28°C for 5 days (A) or at 7°C for 14 days (B).
Previously, injured litchi fruit tissue was observed to increase electrical conductivity due to electrolyte leakage, and was associated with membrane integrity loss (Sivakumar et al., 2010). The observed increase in electrical conductivity for each litchi treatment over storage is displayed in Figure 3A. After 5 days at 28°C and 14 days at 7°C, the electrical conductivities of anoxic treated fruit were significantly (P < 0.05) lower than the control, with the 18 h anoxic treatment yielding the lowest value. A similar result was reported for litchi and loquat fruit, in which short-term anoxic treatment dramatically reduced rises in membrane permeability compared to control fruit (Gao et al., 2009; Liu et al., 2007). These reports noted that short-term anoxic treatments may maintain membrane integrity by delaying superoxide anion emission, thus reducing senescence and quality deterioration. This supports the responsibility of the short-term anoxia treatment for our observation of slowed membrane degradation in litchi fruit. In the present study, the 24-h anoxic treatment of litchi fruit induced electrical conductivity in the pericarp tissues (Fig. 3A), which negatively impacted skin color and browning index during storage (Fig. 1). Jiang et al. (2004b) also noted that litchi fruit exposed to anoxia for 24 h developed disease more rapidly than untreated fruit. Techavuthiporn et al. (2021) found increased ethanol content, a byproduct of anaerobic respiration, in broccoli treated with anoxic conditions for 22–24 h. Thus, excessive anoxic treatment may contribute to litchi pericarp browning. Pericarp browning, which reduces litchi’s appeal, is triggered by water loss or desiccation of the pericarp (Sivakumar et al., 2010). The weight loss of litchi fruit during storage at ambient and low temperatures was dramatically diminished by anoxic treatment (Fig. 3B). Exposure to 18 h anoxia resulted in the least weight loss. Thus, short-term anoxia treatments delay litchi water loss during storage. This delaying of water loss reflects the results of Techavuthiporn and Boonyaritthongchai (2016), who reported, for asparagus exposed to a N2 atmosphere for 8 h at 25°C, that weight loss after 12 days of storage was lower than for untreated spears. The main factor accelerating weight loss may be the higher rate of transpiration through stomata and metabolism (Techavuthiporn and Boonyaritthongchai, 2016). The exterior surface of the litchi fruit pericarp is coated with a lipid layer, essentially a cuticle, which functions as a barrier restricting fruit non-stomatal water loss (Riederer et al., 2015). Furthermore, the litchi exocarp has a thick palisade-like layer of elongated suberized cells directly beneath the epidermis (Sivakumar et al., 2010). The integrity of the fatty acid chains and suberin may relate to the cuticle barrier properties. Thus, maintaining this cuticle layer likely helps minimize transpiration and reduces weight loss.

Electrical conductivity (A) and weight loss (B) of litchi fruit with 6–24 h anoxic treatment and storage at 28°C for 5 days or at 7°C for 14 days. Different lowercase or uppercase letters above bars indicate significant (P < 0.05) differences among anoxic treatments and storage periods, respectively.
During storage, the TSS and TA content of litchi fruit decreased (Table 1). Fruit TSS content after short-term anoxia treatment did not differ significantly (P > 0.05) from the control for storage at 28°C and 7°C. Fruit TA content decreased continuously during storage, but fruit anoxic exposure resulted in a significantly lower TA content after storage at both temperature conditions. Anoxic treatment significantly reduced TA content, possibly due to decreased flux in the citric acid or tricarboxylic acid (TCA) cycle of respiratory metabolism (Nakamura and Noguchi, 2020). Glycolysis CO2 formation is increased with the reduced O2 level of anoxic conditions. Pyruvate also accumulates with glycolytic flux increase, as it is no longer delivered into the TCA cycle due to decreasing pyruvate dehydrogenase activity (Boeckx et al., 2019).
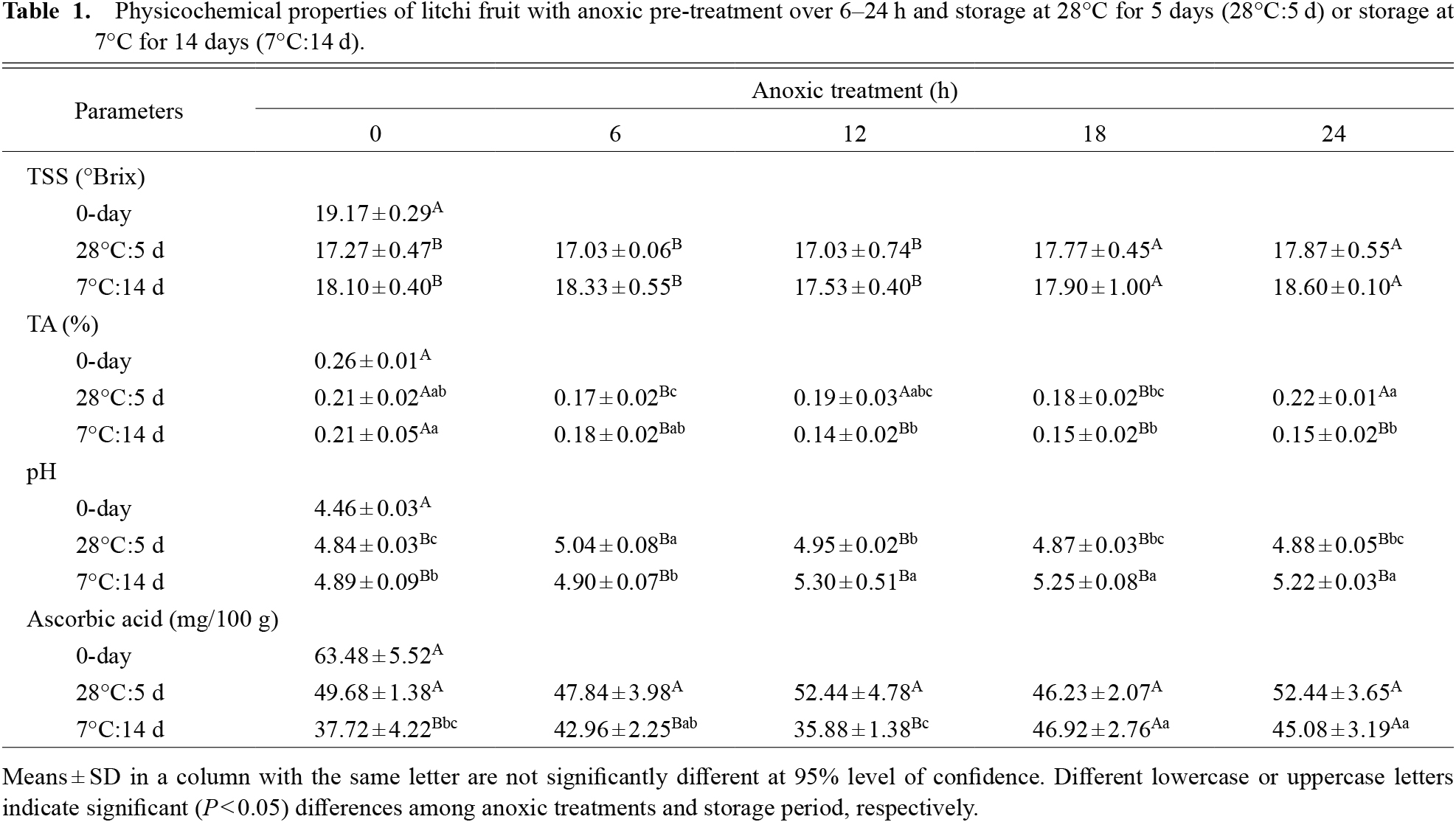
Physicochemical properties of litchi fruit with anoxic pre-treatment over 6–24 h and storage at 28°C for 5 days (28°C:5 d) or storage at 7°C for 14 days (7°C:14 d).
The litchi fruit ascorbic acid content gradually decreased for both storage temperatures of 28°C and 7°C (Table 1). There was no significant difference in litchi fruit ascorbic acid content after storage for 5 days at 28°C. After storage at 7°C for 14 days, the ascorbic acid content of anoxia-treated fruit at 6, 18, and 24 h was significantly (P < 0.05) higher than that of the control and 12 h treated fruit. Our results reflect the report of higher ascorbic content retention for anoxia-treated pineapple (Techavuthiporn et al., 2017), Chinese water chestnut (You et al., 2012), and litchi (Jiang et al., 2004b). Nakamura and Noguchi (2020) reported that under O2-deficient conditions, production of ascorbate/glutathione cycle in the mitochondrial matrix was induced to maintain plant cell redox balance.
Secondary metabolites of litchi fruit during storagePolyphenolics, flavonoids, and anthocyanins, a category of flavonoids, comprise a broad collection of secondary metabolites found in plants. These chemicals function in plants as low molecular mass antioxidants and scavenge excess reactive oxygen species (ROS) (Yun et al., 2021). The oxidation of these chemicals by browning enzymes (PPO and POD) is responsible for the synthesis of the polymeric browning pigment in the litchi pericarp (Sivakumar et al., 2010). During storage, total phenolic content in control pericarp sharply declined after 5 days at 28°C, while phenolic content levels were maintained after 14 days at 7°C (Fig. 4A). After anoxic treatment, the total phenol content was significantly (P < 0.05) higher than that of the control pericarp at ambient temperature. Fruit exposed to anoxic condition for 24 h showed the highest total phenol content, followed by 12, 18, and 6 h, respectively. While anoxic treatment for 6 and 18 h showed higher total phenol accumulation than other treatments and control pericarp for 14 days of cool storage. During postharvest storage, total flavonoid content in control pericarp decreased at 28°C but gradually increased at 7°C (Fig. 4B). After anoxia pre-treatment, total flavonoid content followed the same trend as total phenolic content during storage. As a substrate for browning enzymes, flavan-3-ol derivatives accounted for 96–97% of total litchi pericarp flavonoids, which were reduced upon storage or browning (Reichel et al., 2013; Zhang et al., 2000). In the present study, litchi fruit with anoxic treatments retained higher phenolic compound levels. A similar trend is also reported for anoxically-treated pineapple (Techavuthiporn et al., 2017), kiwi (Song et al., 2009), and Chinese water chestnut (You et al., 2012). The increased retention of total phenolic and flavonoid contents in anoxically-treated fruit may be due to decreased PPO and/or POD activity in comparison to the control fruit.
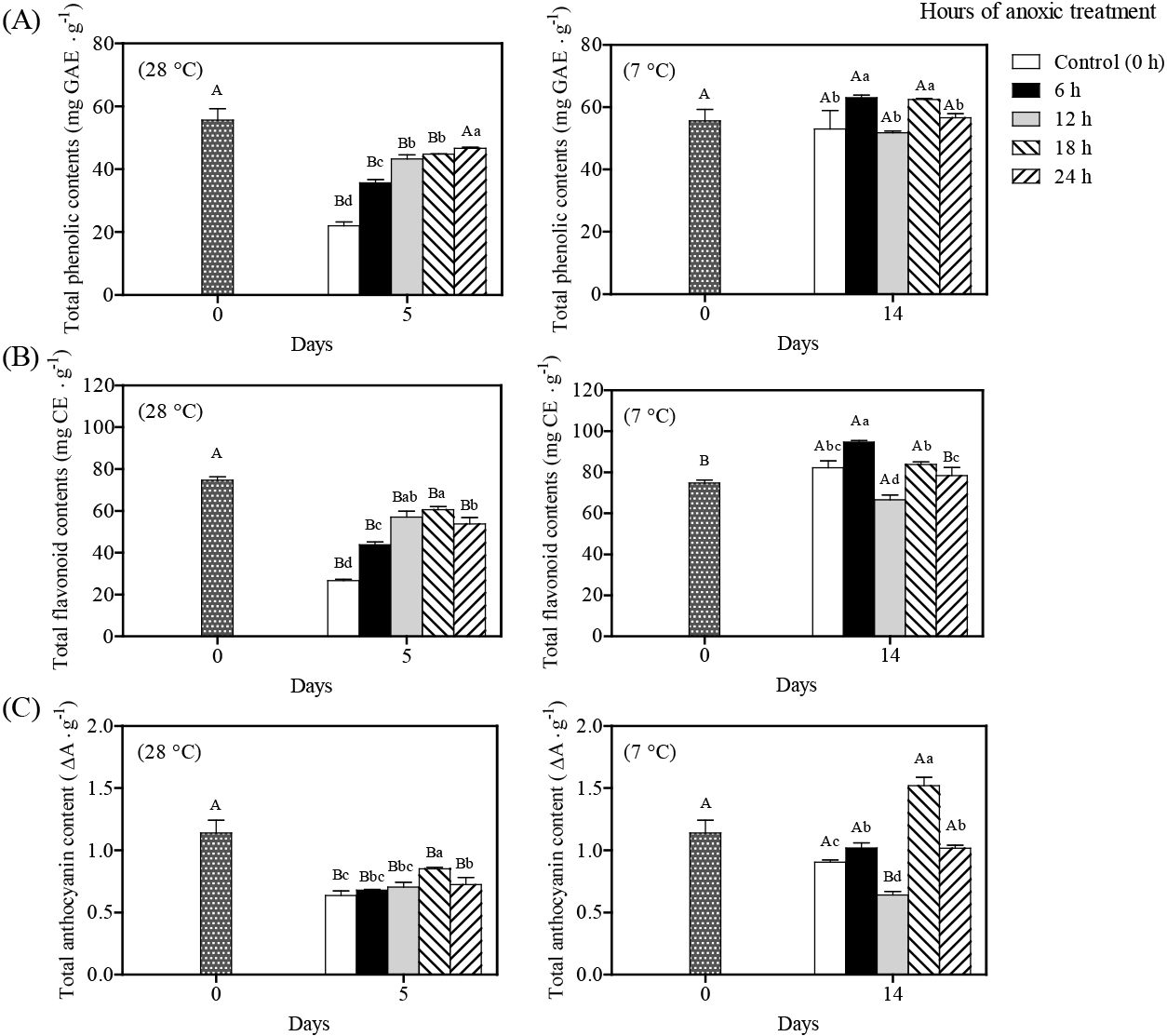
Total phenolic (A), flavonoid (B) and anthocyanin (C) content of litchi fruit with 6–24 h anoxic treatment and storage at 28°C for 5 days or at 7°C for 14 days. Different lowercase or uppercase letters above bars indicate significant (P < 0.05) differences among anoxic treatments and storage periods, respectively.
Regardless of treatment, total fruit pericarp anthocyanin content decreased over storage (Fig. 4C). However, short-term anoxic treatment retained significantly (P < 0.05) higher anthocyanin content than control fruit. Fruit with the 18 h anoxic treatment yielded the highest anthocyanin retention rate during storage at 28°C for 5 days and 7°C for 14 days. Anthocyanin retention was consistent with the color a* value as well as the absence of pericarp browning in fruit treated anoxically for 18 h (Fig. 1A). Anoxic treatment may stimulate anthocyanin accumulation with multiple enzymes influencing litchi anthocyanin production, including phenylalanine ammonialyase (PAL), a crucial flavonoid synthesis enzyme for anthocyanins and lignin. It is reported that short-term anoxia in litchi pericarp is associated with increases in the gene responsible for anthocyanin production, and enhanced PAL activity in anoxia-treated litchi fruit (Zhang et al., 2005). Conversely, fruit pre-treated in anoxic conditions for 12 h showed the lowest anthocyanin content after storage at 7°C for 14 days. In the present investigation, this anthocyanin concentration decrease is related with an increase in browning index.
Antioxidant properties of litchi fruit during storageThe antioxidant capacities of control litchi pericarp decreased dramatically after 5 days of storage at 28°C, as measured by the DPPH and FRAP assays, while capacities were maintained during storage at 7°C for 14 days (Fig. 5). During storage at 28°C for 5 days, short-term anoxic treatment maintained higher antioxidant levels by DPPH and FRAP assays than in control fruit. However, there was no significant difference in antioxidant activity by the DPPH assay for litchi fruit with anoxic treatment with cool storage. On the other hand, fruit with anoxic treatment for 12 h yielded reduced antioxidant activity by the FRAP assay. This discrepancy between the DPPH and FRAP test findings during cool storage may be attributed to the reaction mechanism. The DPPH assay measures a hydrogen atom transfer rate, whereas the FRAP assay measures a single electron transfer reaction. Generally, the non-enzymatic antioxidant activity (reducing power and free radical scavenging activity) decreased during storage and was associated with the phenolic content (You et al., 2012). In this study, short-term anoxic treatments of litchis delayed reductions in free radical scavenging activity against DPPH and FRAP during ambient storage. A significant correlation was found between DPPH and FRAP activities and total phenolic and flavonoid contents. These substantial relationships imply that phenolics/flavonoids contribute to the litchi fruit pericarp antioxidant capacity. Anthocyanins, due to their positive charges or abundance of hydroxyl groups in the B-ring, have stronger antioxidant activity than other flavonoids (Liu et al., 2018).
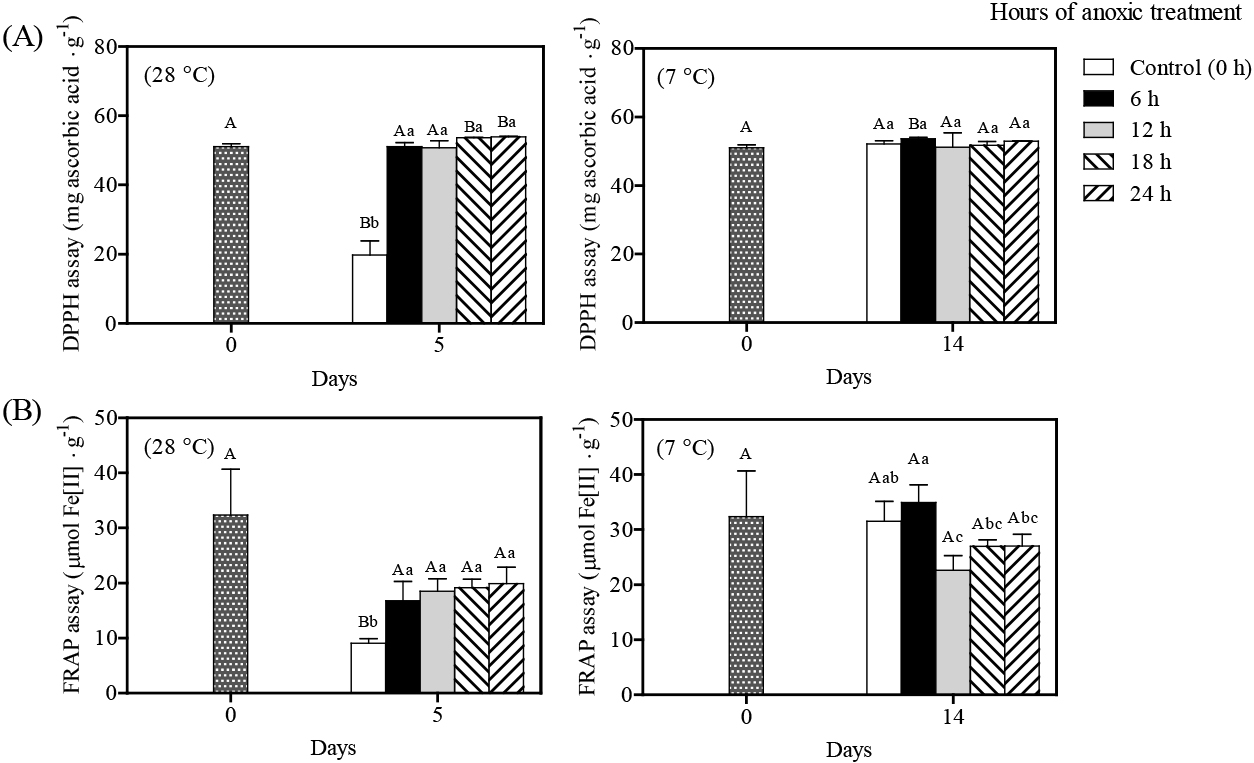
Antioxidant activity by DPPH (A) and FRAP (B) assay of litchi fruit with 6–24 h anoxic treatment and storage at 28°C for 5 days or at 7°C for 14 days. Different lowercase or uppercase letters above bars indicate significant (P < 0.05) differences among anoxic treatments and storage periods, respectively.
Three essential components contribute to enzymatic browning: O2, phenolic chemicals, and PPO (Li et al., 2022). In the present study, a decline in PPO activity was observed in litchi stored at 28°C (Fig. 6A). This observation is supported by the findings of Sivakumar and Korsten (2010) and Reichel et al. (2013). In contrast, higher PPO activity was observed compared with the initial day (0-day) with cool storage. Nevertheless, the total phenolic content in the anoxically-treated pericarp was not significantly different compared to the control pericarp for cool storage compared with the initial content (0-day). Wang et al. (2014) reported a pattern of PPO activity in litchi pericarp at different temperatures, which rapidly increased at the beginning of storage but decreased afterwards. It is conceivable that the low temperature storage steadily declined enzymatic activities. Anoxic treatment for 12–24 h in litchi fruit limited increases in PPO activity during storage at 7°C, but the reduction in PPO activity was considerably reduced for storage at 28°C. This may be due variations in the effect of anoxic treatment due to exposure period and storage temperature, which require further examination. Zhang et al. (2005) reported that litchi PPO activity was inconsistent with browning degree during storage. Previous research on litchi pericarp browning indicated POD and PPO activity involved in anthocyanin degradation (Liu et al., 2018; Zhang et al., 2005). As PPO cannot directly oxidize litchi anthocyanin, it is quickly sequestered forming an anthocyanin-PPO-phenol complex (Zhang et al., 2005). Our study on litchi revealed that increased POD activity in the control pericarp was consistently associated with browning index during storage at both storage temperatures, and negatively associated with anthocyanin, total phenolic, and flavonoid contents (Fig. 6B). During storage, anoxia-treated litchi yielded lower POD activity than control fruit. However, POD activity in fruit treated anoxically for 12 h yielded the highest level for cool storage, which corresponded with skin browning index and anthocyanin content. These findings confirm the involvement of POD in litchi enzymatic browning and anthocyanin breakdown in litchi pericarp.

Pericarp browning enzyme, PPO (A) and POD (B) activities of litchi fruit with 6–24 h anoxic treatment and storage at 28°C for 5 days or at 7°C for 14 days. Different lowercase or uppercase letters above bars indicate significant (P < 0.05) differences among anoxic treatments and storage periods, respectively.
Short-term anoxic treatment significantly slowed pericarp browning and preserved fruit quality in litchi. After litchi fruit storage for 5 days at 28°C and 14 days at 7°C, the anoxic treatment over 18 h resulted in the best color retention, whereas 12 h was the least effective. Higher levels of anthocyanins, phenolics, and flavonoids were observed in anoxically-treated litchi fruit, which demonstrated the treatment enhancing non-enzymatic antioxidant activity. After anoxic treatment, the non-enzymatic antioxidant system along with the lower POD activity may delay pericarp browning, thus maintain litchi fruit quality.
The quality of harvested litchi cv. Hong Huey was sustained at ambient and cool temperatures after a short anoxic treatment. Based on the findings of this study, 6 h anoxic treatment before storage appears optimal from both a produce quality and economic standpoint. This chemical-free and low-cost technique merits further exploration, particularly for applications in commercial distribution systems with inadequate refrigeration.
The authors would like to acknowledge the Plant Genetic Conservation Project Under the Royal Initiation of Her Royal Highness Princess Maha Chakri Sirindhorn (RSPG) and the Thailand Science Research and Innovation Fund, University of Phayao [Grant No. FF66-UoE012] for the financial support.