2023 年 92 巻 3 号 p. 261-268
2023 年 92 巻 3 号 p. 261-268
Evaluating the function of genes expressed in fruit tissues of fruit tree species using a genetic transformation approach is a long process because the trees are generally recalcitrant to genetic transformation and cannot bear fruit during their long juvenile phases. Transient gene expression in fruit enables the functional analysis of genes associated with fruit traits, which may accelerate the study of fruit physiology. Here, by using the recently developed “Tsukuba system”, we successfully established an efficient transient expression system in harvested fruit tissues. The “Tsukuba system” utilizes a combination of the geminiviral replication system and a double terminator, which ensures sufficient levels of transgene expression. We used blueberry fruit as a model to characterize the applicability of this system for transient expression in fruit tissue. The pTKB3-EGFP vector was introduced by agroinfiltration into the fruit tissues of several blueberry cultivars. We found that transient GFP fluorescence in fruit peaked 4–6 days after agroinfiltration. Agrobacterium suspensions were easily injected into soft, mature fruit, and GFP was strongly expressed; however, hard, immature fruit were not penetrable by Agrobacterium suspensions, and GFP was rarely detected. We then tested the applicability of the developed system to other fruit tree species: six families, 17 species, and 26 cultivars. GFP fluorescence was detected in all species, except for Japanese apricot. In blueberry, bilberry, sweet cherry, apricot, and satsuma mandarin, GFP was highly expressed and observed in a large proportion of the flesh. In kiwifruit, hardy kiwifruits, persimmon, peach, apple, European pear, and grape, GFP fluorescence was limited to certain parts of the fruits. Finally, transient VcMYBA1 overexpression in blueberry was tested as a model for gene functional analysis in fruit. Transient VcMYBA1 overexpression induced red pigmentation in the flesh, suggesting that VcMYBA1 expression caused anthocyanin accumulation. This study provides a technical basis for the rapid evaluation of genes expressed in fruit, which will be useful for gene function evaluation studies in fruit crops with long juvenile phases.
Many genes involved in fruit traits have been isolated in recent years (Zahid et al., 2022), but their functions have not been fully characterized using genetic approaches because it is a lengthy process to evaluate gene functions in fruit trees using mutants (mutagenesis) or stable transformants. Moreover, many fruit tree species are recalcitrant to genetic transformation; in such species, a transient expression system could be an alternative way of evaluating gene functions. Transient transgene expression in fruit has been reported in several species, including strawberry (Fragaria × ananassa), banana (Musa acuminata), kumquat (Fortunella crassifolia), apple (Malus × domestica), and grapevine (Vitis labrascana) (Gong et al., 2021; Li et al., 2021a; Lv et al., 2019; Shan et al., 2020; Zhao et al., 2019). However, transient expression in fruit has not always been successful because it is difficult to ensure sufficient expression levels for functional analysis.
A high-level expression system for recombinant proteins called the “Tsukuba system” has recently been developed (Nosaki et al., 2021a). Vectors for the Tsukuba system harbor the geminiviral replication system to ensure rolling circle replication and a double terminator composed of a heat shock protein and extensin terminators to efficiently terminate transcription (Yamamoto et al., 2018). When the Tsukuba system was adapted for ectopic gene expression in Nicotiana benthamiana, protein expression levels reached 4 mg·g−1 fresh weight, which was higher than that obtained with the magnICON system, the most well-known deconstructed viral vector (Yamamoto et al., 2018). The Tsukuba system has also been applied to several crops, including tomato (Solanum spp.), eggplant (S. melongena), hot pepper (Capsicum frutescens), melon (Cucumis melo), orchid (Phalaenopsis aphrodite), soybean (Glycine max), Lotus japonicus, common bean (Phaseolus vulgaris), and radish (Raphanus sativus) (Hoshikawa et al., 2019; Kitajima et al., 2020; Yamamoto et al., 2018). However, the Tsukuba system has not been tested in fruit tissues of fruit tree crops. In the present study, we tested the applicability of this system for transient expression using many different fruit trees from different plant taxa. To the best of our knowledge, this is the first report comparing the efficiency of transient expression in fruits from many species. By using various fruit species, we determined the key factors affecting efficient transient expression and demonstrated that this system can be applied to functional studies of key genes expressed in fruit. This study provides valuable information to accelerate future functional studies of genes related to fruit development, quality, and ripening.
In this study, six families, 17 species, and 26 cultivars were used (Table 1). Actinidia fruits were harvested from trees grown in the experimental field of the Faculty of Agriculture, Kagawa University, Sanuki, Japan (34° N, 134° E). ‘Shine Muscat’ grapes were harvested from field-grown trees at the Kizu experimental farm, Kyoto University, Kizugawa, Japan (34° N, 135° E). All the other fruits were harvested from field- and pot-grown trees at the experimental farm of Kyoto University, Kyoto, Japan (34° N, 135° E).
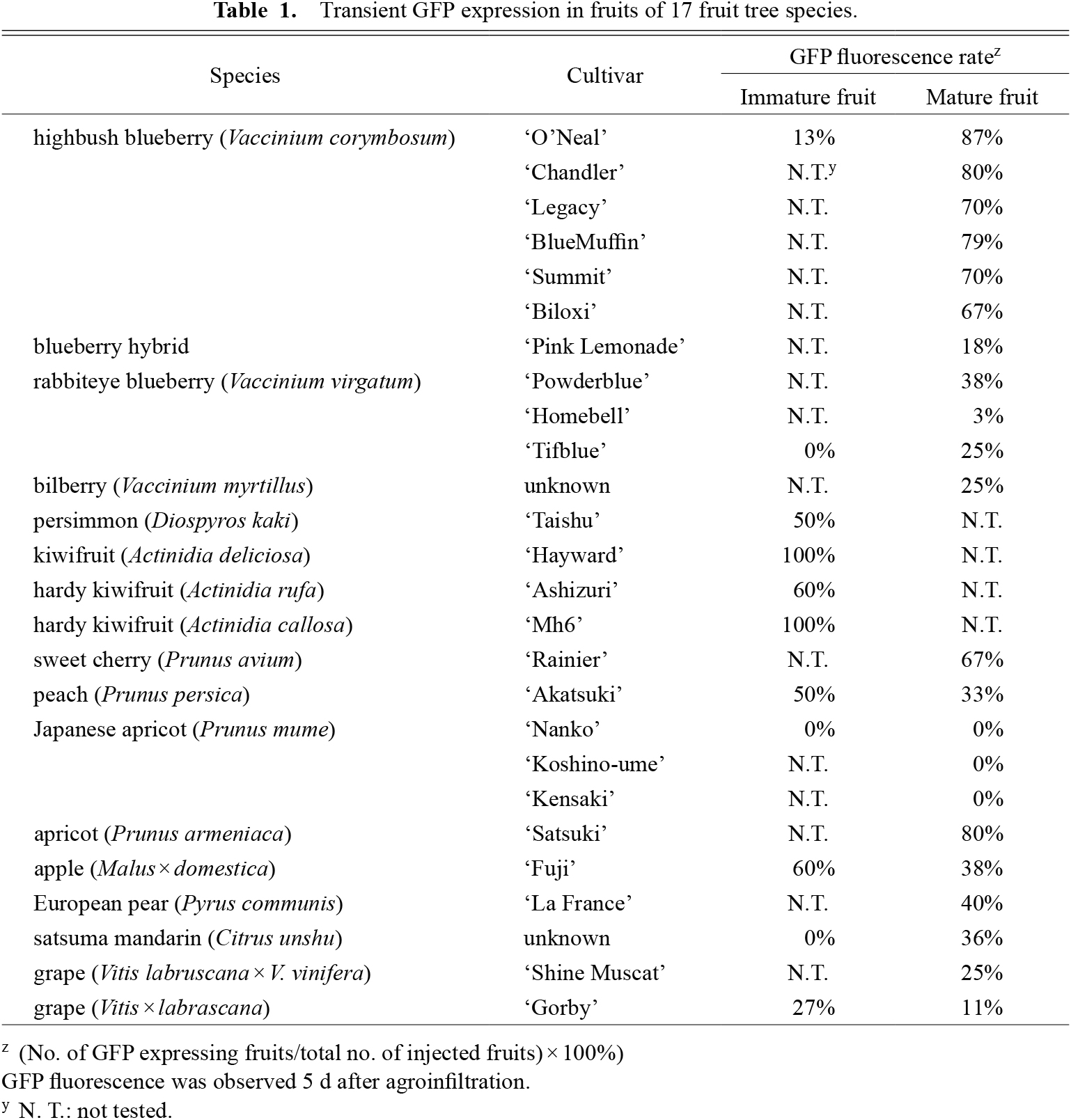
Transient GFP expression in fruits of 17 fruit tree species.
The full-length coding sequence of VcMYBA1 (VaccDscaff1486-snap-gene-0.3) was amplified from ‘O’Neal’ blueberry leaf gDNA using three primer sets corresponding to its three exons. The primers for cloning VcMYBA1 are listed in Supplementary Table S1. Cloned VcMYBA1 was introduced into Sal1-digested pTKB3 by the In-Fusion method (In-Fusion HD Cloning Kit, TaKaRa Bio, Inc., Shiga, Japan). pTKB3 is one of the Tsukuba system vectors customized for efficient transient expression in plant tissues. pTKB3 contains a gene-silencing suppressor (p19), a geminiviral replication system, a double terminator composed of a heat shock protein and extensin terminators, the cauliflower mosaic virus (CaMV) 35S promoter with a double-enhanced element, and tobacco mosaic virus Ω sequence (TMV Ω) for a 5' untranslated region (UTR). These components of the pTKB3 enable a high level of transient expression (Fig. 1; Nosaki et al., 2021a; Yamamoto et al., 2018). The VcMYBA1 sequence was placed under the 35S promoter. The resultant pTKB3-VcMYBA1 plasmid was shipped to Eurofins Genomics (Tokyo, Japan) and sequenced by the Sanger sequencing method using a 3730xl DNA Analyzer (Thermo Fisher Scientific, Waltham, MA, USA).
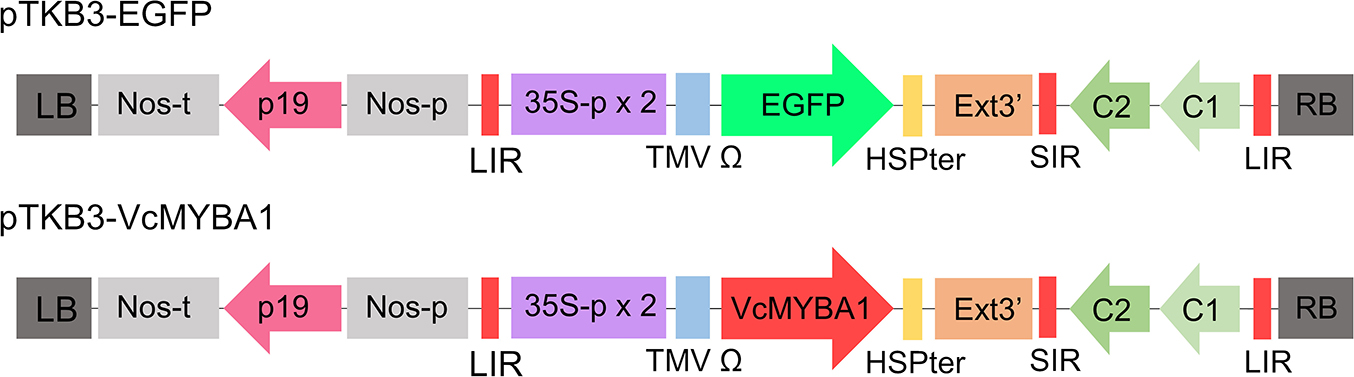
Schematic diagram of the T-DNA region of the vectors used in this study. 35S-p x 2: CaMV 35 S promoter with double-enhanced element, TMV Ω, 5'-leader sequence of tobacco mosaic virus, EGFP: enhanced green fluorescence protein, VcMYBA1: blueberry MYBA1 (VaccDscaff1486-snap-gene-0.3), HSPter: terminator of heat shock protein gene, Ext3': tobacco extension gene 3' element, LIR: long intergenic region of bean yellow dwarf virus (BeYDV) genome, SIR: short intergenic region of BeYDV genome, C1/C2: BeYDV ORFs C1 and C2 encoding for replication initiation protein (Rep) and RepA, respectively; LB and RB, the left and right borders of the T-DNA region, respectively; Nos-p and Nos-t, NOS promoter and terminator, respectively; and p19: a gene-silencing suppressor gene from tomato bushy stunt virus.
The pTKB3-EGFP (Nosaki et al., 2021a) and pTKB3-VcMYBA1 vectors (Fig. 1) were introduced into Agrobacterium tumefaciens strain EHA105 cells by electroporation (MicroPulser Electroporator; Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions. Agrobacterium cells containing the vectors were grown for 24–48 h at 28°C in liquid LB medium supplemented with 50 mg·L−1 kanamycin and 10 mg·L−1 rifampicin. After centrifugation at 1,644 × g for 15 min, the resulting pellet was resuspended in liquid co-cultivation medium [MW basal medium (Tetsumura et al., 2008) supplemented with 20 g·L−1 sucrose, 20 mg·L−1 acetosyringone, 10 mM MgCl2, and 10 mM 2-(N-morpholino) ethane-sufonic acid (MES) (pH 5.6)]. The MW medium comprised equal volumes of MS (Murashige and Skoog, 1962) and WPM (McCown and Lloyd, 1981) media. The Agrobacterium solution was diluted to an optical density of 0.5 at 600 nm and incubated at 28°C for 5 h. Agroinfiltration was performed using a 1-mL syringe with a 0.70 × 25-mm needle (Fig. 2A). The suspension solution was injected gradually as the needle was inserted into the center part of the fruit. The volume of the injected solution ranged from 0.1–1.0 mL; 0.1–0.2 mL for blueberries (Vaccinium corymbosum and V. virgatum), bilberry (V. myrtillus), hardy kiwifruits (A. rufa and A. callosa), and sweet cherry (Prunus avium), 0.3–0.5 mL for persimmon (Diospyros kaki), kiwifruit (A. deliciosa), Japanese apricot (P. mume), apricot (P. armeniaca), immature apple, satsuma mandarin (Citrus unshu), and grapes (Vitis labruscana × V. vinifera, V. × labrascana), and 1 mL for mature apple and European pear (Pyrus communis). At least five fruits from each cultivar were harvested and infiltrated. The harvest dates for each fruit are listed in Supplementary Table S2. Injected fruits were maintained at room temperature (22–28°C) and with a 10–13 h photoperiod (20–30 mE·m−2·s−1 from cool white fluorescent tubes). The expression of GFP in fruits was detected under blue light (488 nm) using a fluorescence microscope (MVX10-2/KTKS; Olympus, Tokyo, Japan) equipped with a mercury lamp (BH2-RFL-T3; Olympus).

Transient GFP expression in blueberry. (A) Agroinfiltration into blueberry fruit. The suspension was prepared in a 1-mL syringe with a needle. The solution was injected gradually as the needle was inserted into the center part of the fruit. (B) GFP expression detected under blue light 5 d after injection. ‘Blue Muffin’ highbush blueberries were injected with Agrobacterium harboring pTKB3-EGFP or MW liquid co-cultivation medium. Red autofluorescence from chlorophyll can be observed under blue light. (C) Periodic observation of GFP fluorescence in ‘O’Neal’ blueberry. All the fruits were injected with Agrobacterium at the position of the white arrow after harvesting. (D) GFP fluorescence in green, immature ‘O’Neal’ fruit and ripe blueberry fruit 5 d after agroinfiltration. (E) Comparison of GFP fluorescence in highbush blueberry (‘O’Neal’ and ‘Legacy’) and rabbiteye blueberry (‘Homebell’ and ‘Tifblue’). GFP fluorescence was observed 5 d after agroinfiltration. (F) The internal appearance of highbush blueberry ‘Legacy’ and rabbiteye blueberry ‘Homebell’. (Agro) represents agroinfiltration, (MW) represents injection with MW liquid co-cultivation medium. All fruits were observed 5 d after injection. No fruits showing watercore or red pigmentation expressed GFP.
GFP expression was observed in ‘Blue Muffin’ highbush blueberry 5 d after injection. Strong GFP was detected under blue light in fruits injected with Agrobacterium harboring pTKB3-EGFP while no GFP expression was observed in fruits injected with the MW liquid co-cultivation medium (Fig. 2B). Next, we periodically observed GFP fluorescence in ‘O’Neal’ highbush blueberry fruit from 1 h to 10 d after injection (Fig. 2C). GFP fluorescence in ‘O’Neal’ fruits was not observed 1 h to 2 d after agroinfiltration. Weak GFP fluorescence could be detected in fruits 3 d after agroinfiltration, but GFP fluorescence was the most intense at 4–6 d; after 6 d, the fluorescence intensity declined. Because GFP fluorescence was not observed immediately after agroinfiltration (Fig. 2C), this indicated that GFP was produced from the plant cells rather than Agrobacterium. Based on this result, 5 d after agroinfiltration was considered optimal timing for observing GFP fluorescence in blueberry fruit; therefore, we observed GFP fluorescence at 5 d. GFP fluorescence was much stronger in ripe blueberries than in green ones (Fig. 2D). We also compared GFP fluorescence among six highbush blueberry (V. corymbosum) cultivars, three rabbiteye blueberry (V. virgatum) cultivars, and one hybrid cultivar (Fig. 2E; Table 1). Highbush blueberries tended to exhibit stronger GFP fluorescence than rabbiteye and hybrid blueberries. More than 60% of fruits of the highbush blueberry cultivars exhibited GFP fluorescence, but less than 40% of rabbiteye blueberry fruits did so. The highest GFP fluorescence rate was recorded for the highbush blueberry ‘O’Neal’ (87%), whereas GFP fluorescence was rarely observed in the rabbiteye blueberry ‘Homebell’ (3.3%). In ‘Homebell’ fruits, we often detected watercore and/or red pigmentation in the flesh after agroinfiltration, which may have caused less or no GFP fluorescence (Fig. 2F). Few highbush blueberries showed watercore or pigmentation after agroinfiltration (Fig. 2F). Watercore and red pigmentation occurred in some ‘Homebell’ fruits, even after injection of MW liquid co-cultivation medium or without injection (Fig. 2F), indicating that these physiological changes often occurred in ‘Homebell’ independent of Agrobacterium infection.
Transient GFP expression in fruits of 17 fruit tree speciesTo test the applicability of the transient expression system in other plant species, agroinfiltration with pTKB3-EGFP was performed in fruits from various fruit species (Table 1; Fig. 3). In blueberry, bilberry, sweet cherry, apricot, and satsuma mandarin, strong GFP fluorescence was detected. In persimmon, kiwifruit, hardy kiwifruit, peach, apple, European pear, and grapes, only weak GFP fluorescence was observed, or GFP fluorescence sites were limited to some parts of the fruits. In Japanese apricot, no GFP fluorescence was detected in the three cultivars tested. GFP fluorescence sites differed depending on the species, which was probably related to the internal structures of the fruits. In apple, European pear, and persimmon, injection with Agrobacterium suspension into the flesh was difficult, but when the needle was inserted into the fruit center, injection was easy, which suggested that there was an empty space in the center part of the fruit that could be filled with Agrobacterium suspension. In blueberry, bilberry, sweet cherry, and satsuma mandarin, GFP was expressed throughout the flesh (mesocarp). In persimmon, weak GFP fluorescence was observed in the upper part of the fruit, just below the epidermis. In Actinidia plants, GFP fluorescence was radially spread across the endocarp, particularly within the locules. Immature fruits of Actinidia plants could not be injected with Agrobacterium suspension, unless it was injected from the bottom of the fruit, and GFP fluorescence was restricted to the mesocarp. Even when Agrobacterium solution was injected into the white central core (axile placentation) or mesocarp of Actinidia fruits, GFP fluorescence was only observed in the locules of the fruits, suggesting that the Agrobacterium suspension may have only spread to the locules. In peach, weak GFP fluorescence was observed only near the needle insertion site in both immature and mature fruits. In apricot, strong GFP fluorescence was observed in the flesh around the endocarp (Fig. S1). In immature apple, GFP was strongly expressed in seeds, whereas the flesh did not exhibit any GFP fluorescence. In mature apple and European pear, weak GFP was expressed in the seedcoats, but none was detected in the flesh. In satsuma mandarin, GFP was observed throughout the whole flesh. In grapes, GFP fluorescence was detected only in the endocarps of both immature and mature fruits (Fig. S2). Overall, GFP fluorescence was detected in 16 of 17 fruit species, which suggested that our transient expression system was robust and could be applicable to other fruit species.
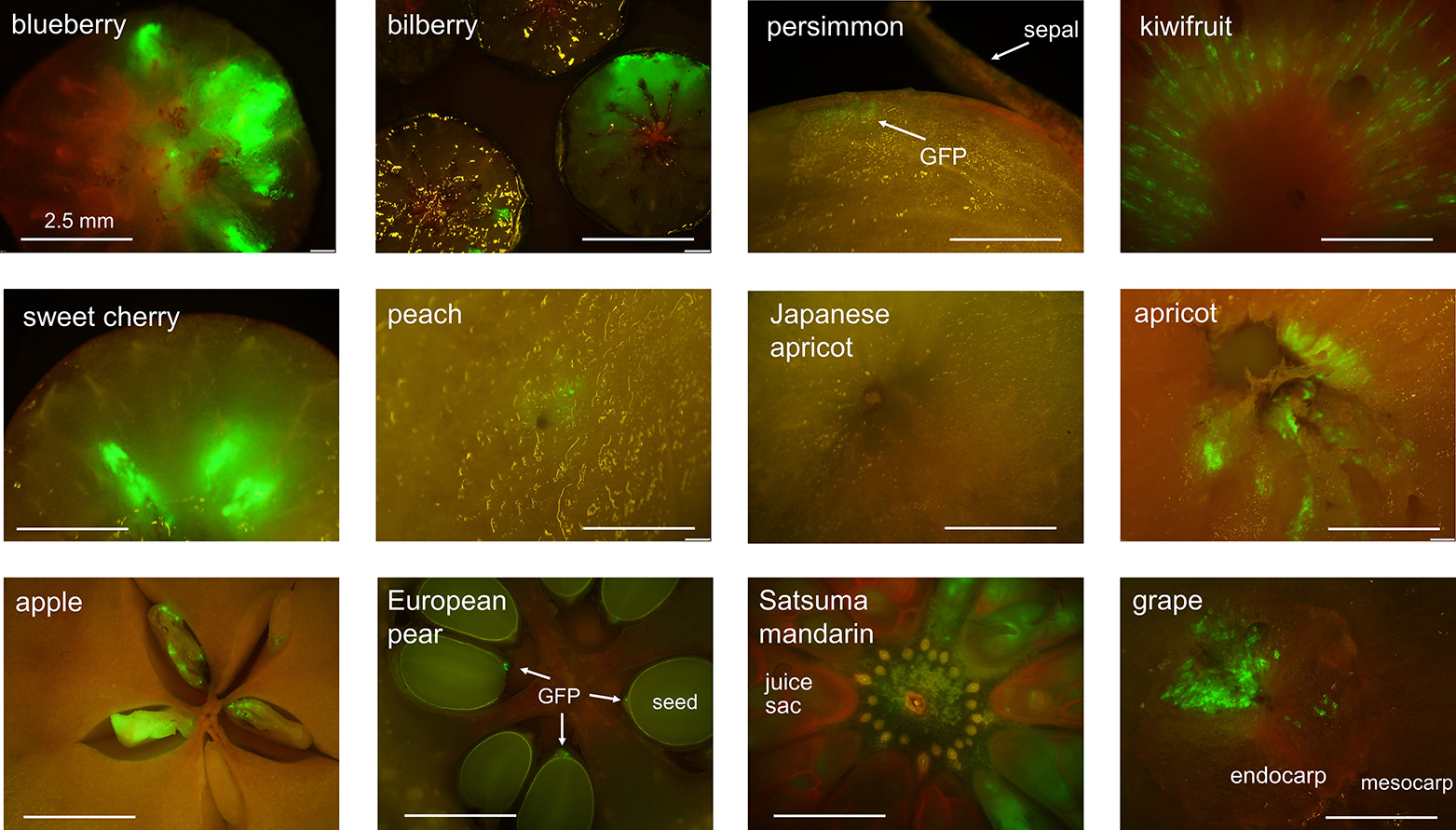
Transient GFP fluorescence in various fruits. GFP fluorescence was observed 5 d after agroinfiltration in mature blueberry (‘O’Neal’), mature bilberry, immature persimmon (‘Taishu’), immature kiwifruit (‘Hayward’), mature cherry (‘Rainier’), mature peach (‘Akatsuki’), mature Japanese apricot (‘Nanko’), mature apricot (‘Satsuki’), immature apple (‘Fuji’), mature European pear (‘La France’; GFP fluorescence is indicated by the white arrows), mature satsuma mandarin, and immature grape (‘Gorby’). Harvesting dates for each fruit are listed in Supplementary Table S2.
VcMYBA1 is a key MYB transcription factor involved in anthocyanin biosynthesis (Lafferty et al., 2022a, b; Li et al., 2021b; Wu et al., 2022). When pTKB3-VcMYBA1 was injected into the highbush blueberry ‘Summit’ by agroinfiltration, we detected red pigmentation around the vascular area, and coloration was stronger over a larger area than in controls (Fig. 4). This result suggested that VcMYBA1 could be successfully transiently expressed in blueberry flesh, which may induce anthocyanin accumulation.

Transient VcMYBA1 expression in ‘Summit’ highbush blueberry. ‘Summit’ highbush blueberry was harvested on 14 July. Agrobacterium containing pTKB3-EGFP or pTKB3-VcMYBA1 was injected into blueberry after harvest. Injected blueberries were observed under blight field using the fluorescence microscope 5 d after injection. Two representative fruits for each treatment were shown.
With the vast amount of available omics data, many candidate genes associated with important agronomic traits have been identified in recent years. Simultaneously, the demand for rapid and effective tools to determine gene function has increased. Transient protein expression is a promising strategy to validate novel gene functions quickly and efficiently. In this study, we successfully demonstrated that the recently developed vector, the Tsukuba system, could be efficiently used for transient expression in fruit tissues of different fruit tree species. This is the first report that shows the applicability of the Tsukuba system in fruit tissues across family-wide species. We found the efficiency of the transient expression system varied depending on the species and fruit developmental stage. Here, we examine physical, chemical, and biological aspects that underpin successful Agrobacterium-mediated transient expression in fruits using this system.
Physical factorsThe first prerequisite for successful transient expression is uptake of the Agrobacterium suspension by the fruit. It was difficult to inject the Agrobacterium solution into immature fruits because of their hard and dense flesh. However, injections were successful in all mature fruits. The location of GFP fluorescence differed across species, which may be due to differences in internal structures among them. For example, in apple and European pear, it was difficult to inject liquid into the flesh because it was hard, and there was no available space for the Agrobacterium suspension. However, it was easy to inject liquid into the center of these fruits because of empty spaces around the seeds.
Furthermore, GFP fluorescence in seeds of immature apples was much stronger than that of mature apple (Fig. S3), suggesting that immature seeds were soft and easily infected by Agrobacterium.
Chemical factorsAlthough all the mature fruits were easily injected with the Agrobacterium suspension, no GFP fluorescence was observed in Japanese apricot. The pH of Japanese apricot is generally much lower than other fruits, around pH 2.0–3.0, even when mature (FDA, 2007; Kim et al., 2014; Shim et al., 1989). Agrobacterium EHA101 growth was vigorous at pH 5.0–7.0, but no growth was observed at pH 4.5 (Ogaki et al., 2008). EHA105 used in this study was generated from EHA101 through site-directed deletion of the kanamycin resistance gene from the Ti plasmid; otherwise, these two are identical (Hood et al., 1993), suggesting EHA105 as well as EHA101 growth can be restricted by a low pH condition. Therefore, fruit pH may also affect the applicability of agroinfiltration, and the low pH in Japanese apricot may be why transient expression failed.
Fruits are generally richer in phytoalexins, such as polyphenols, than leaves, which are usually used for stable transformation. From this perspective, the inside of fruit may be a harsher environment for Agrobacterium than that in leaves. Plants produce phenolic compounds when exposed to stress and pathogen attack (Clé et al., 2008; Schmitz-Hoerner and Weissenbock, 2003), and various polyphenols inhibit bacterial growth (Daglia, 2012). If these antibacterial chemicals are factors preventing transient expression in fruit, injection of Agrobacterium together with polyphenol adsorbents (e.g., polyvinylpyrrolidone (PVP) and charcoal) may be effective.
Biological factorsAgrobacterium infection and plant defense responses are closely related (Pitzschke, 2013; Tiwari et al., 2022). The hypersensitive response in plants in reaction to Agrobacterium infection often results in necrosis and poor survival rates of transformed plant tissues (Khanna et al., 2007; Kuta and Tripathi, 2005). The type of protein expressed can also cause cell death in plants and reduce the expression of recombinant proteins (Nosaki et al., 2021b). Thus, the viability of plant cells and tissues after Agrobacterium infection is also an important factor in efficient transient expression. Once plants perceive signals of Agrobacterium invasion, they overproduce reactive oxygen species (ROS), leading to enhanced expression of defense response-related genes, accumulation of antimicrobial substances, and cell death (Kuta and Tripathi, 2005). In the rabbiteye blueberry, ‘Homebell’, which exhibited little GFP fluorescence, most of the fruits became watercored after injection, and the flesh showed red pigmentation, which was probably derived from anthocyanin accumulation. ‘Homebell’ developed necrosis after injection without Agrobacterium, but it was more severe after Agrobacterium injection. Other blueberry cultivars also had no GFP fluorescence in fruit with watercore. In plant species that have an excessive defense response to Agrobacterium injection that can result in the necrosis of plant tissues, transient expression can be increased by using an improved Agrobacterium, which can suppress plant host defense responses (Raman et al., 2022), or antioxidants such as ascorbic acid, which can suppress the action of ROS (Nosaki et al., 2021b).
Utility of Tsukuba system for evaluation of genes expressed in fruitTransient VcMYBA1 overexpression in blueberry was tested as a model case of functional analysis using the Tsukuba system in fruit. As expected, VcMYBA1 overexpression induced red pigmentation in the flesh, which was consistent with a previous report suggesting that VcMYBA1 induces anthocyanin accumulation by activating anthocyanin biosynthetic genes in tobacco plants (Li et al., 2021b). Although transgenic blueberry overexpressing VcMYBA1 accumulates anthocyanins through upregulation of anthocyanin biosynthetic genes (Lafferty et al., 2022b), the function of VcMYBA1 in fruit has yet to be characterized. Our transient expression analysis indicated that VcMYBA1 could function as a promoter of anthocyanin biosynthesis in blueberry fruit. Recent genomic and transcriptomic analyses of blueberry have identified many candidate genomic regions responsible for important agronomic traits, such as fruit firmness, fruit weight, pH, soluble solids content, epicuticular wax, and volatile organic compounds (Cappai et al., 2018, 2020; Edger et al., 2022; Ferrão et al., 2018, 2020; Qi et al., 2019). The identification of genes associated with fruit traits in fruit crops other than blueberry has also progressed with the widespread use of high-throughput sequencing technologies (Zahid et al., 2022). Transformation of model plants, such as Arabidopsis or tobacco, is often insufficient to obtain a detailed understanding of the function of genes related to fruit traits because genes may behave differently when expressed in different species or organs. Because of the difficulty involved in transformation and the need to transform the juvenile phase of fruit tree crops, which results in a long wait prior to analysis, our transient expression system may be the optimal method to elucidate the function of genes controlling fruit traits. Our transient expression system can also be applied to the investigation of the transcriptional activity of promoters and subcellular localization of proteins in fruits.
We thank Candace Webb, Ph.D., from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.