2023 年 92 巻 4 号 p. 493-499
2023 年 92 巻 4 号 p. 493-499
There are light-yellow flower cultivars of sweet pea (Lathyrus odoratus L.), but no deep-yellow ones. Observation of sweet pea flowers under ultraviolet and blue light suggested that carotenoids are responsible for the light-yellow coloration. We carried out both spectroscopic and high-performance liquid chromatography (HPLC) analyses of floral extracts of a light-yellow cultivar, ‘Artemis’, and revealed that the responsible pigments are not flavonoids, but carotenoids, among which acylated lutein is a major component. Because lutein is the pigment responsible for the deep-yellow color of flowers in other plants, we expect to be able to generate deep-yellow flower cultivars of sweet pea. The R and C loci are complementary genes regulating biosynthesis of anthocyanin pigments responsible for the cyanic coloration in sweet pea flowers. In progenies obtained by crossing ‘Artemis’ and a white cultivar, ‘Diana White’, whose genotype is RRcc, the F1 plants had a red flower phenotype only, and the F2 plants had four coloration phenotypes that were white, yellow, red and a combination of yellow and red. Furthermore, the F1 plants, obtained by crossing ‘Artemis’ and another yellow cultivar, ‘Stella’, had a combination of yellow and red flower phenotype only. These data indicate that the genotype of ‘Artemis’ is rrCC and the yellow coloration trait is regulated by a single recessive gene, y, and furthermore, that the y gene is not in linkage with the R or C allele. The theoretical segregation ratio of the F2 plants’ phenotypes obtained by crossing ‘Artemis’ as one parent in the case that Y, R and C loci are independent of each other are presented. We also found some cyanic color cultivars containing higher amounts of carotenoids than ‘Artemis’.
Sweet pea (Lathyrus odoratus L.) is an annual vinous plant belonging to the Fabaceae family. Sweet pea is used as a cut flower in Japan, while it is often used as a garden plant in European countries. Flower coloration is one of the most important characteristics of ornamental plants. Sweet pea has a wide range of flower colors including white, pink, reddish purple, and bluish purple, as well as light-yellow. A total of 19 kinds of anthocyanins have been reported as the major cyanic pigments in sweet pea (Harborne, 1960). There are light-yellow flower cultivars of sweet pea, of which the representative cultivar is ‘Artemis’ (Fig. 1A), but no deep-yellow ones have been described. However, the trading volume of artificially dyed deep-yellow sweet pea flowers accounts for 5.6% of the market in Japan (Japan flower promotion center foundation, 2010). This suggests that breeding deep-yellow sweet pea flower cultivars is desirable.
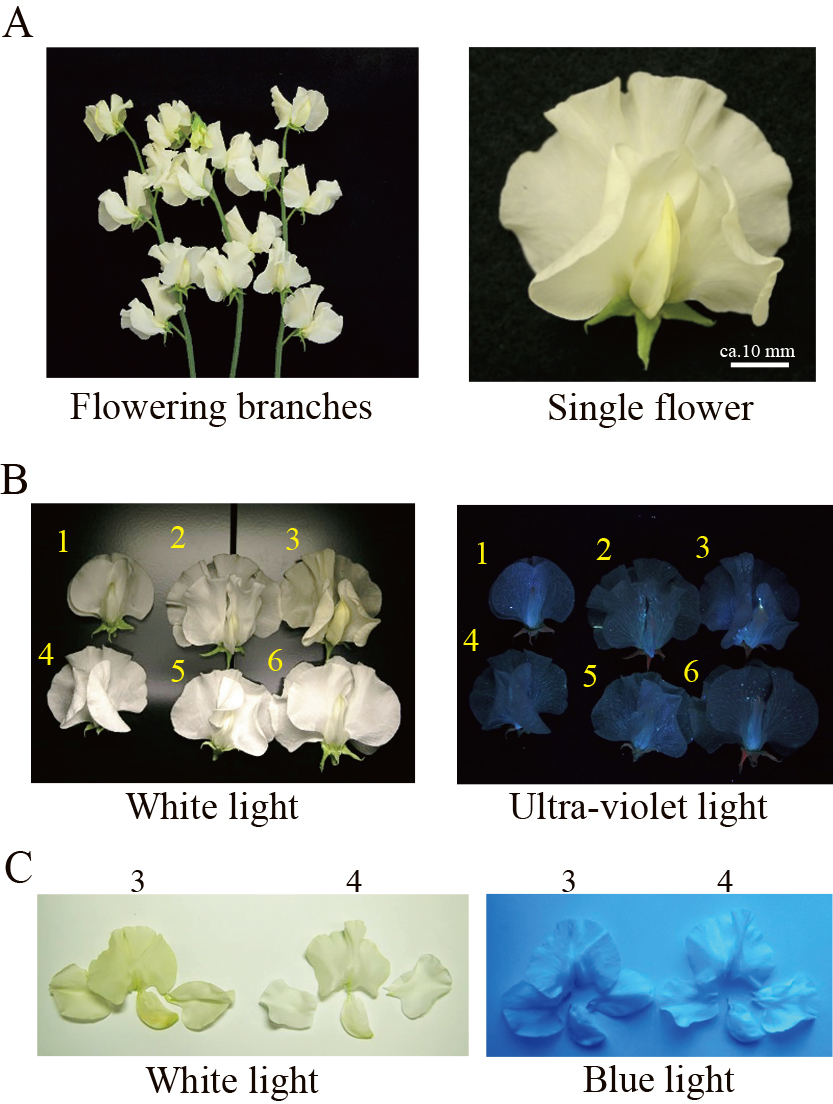
Flower of ‘Artemis’ (A) and observation images of a light-yellow cultivar ‘Artemis’ and white cultivars under white light and (B) ultra-violet light and (C) blue light. 1, ‘Sweet Snow’; 2, ‘Robe Decollete’; 3, ‘Artemis’; 4, ‘Diana White’; 5, ‘Easter Parade’; 6, ‘Com-PurW’ (a F3 plant obtained by crossing between a progeny of ‘367-1’ × ‘Wiltshire Ripple’ and ‘Maggie May’). Images were adjusted to match those of original images.
While deep-yellow coloration of rose, chrysanthemum or pansy flowers is due to carotenoids (Eugster and Märki-Fischer, 1991; Kishimoto et al., 2004; Molnár and Szabolcs, 1980; Molnár et al., 1985), the light-yellow coloration of carnation, cyclamen or snapdragon flowers is due to specific flavonoids of which chromophores are chalcone or aurone (Markham and Ofman, 1993; Mato et al., 2000). It was believed that the light-yellow coloration of sweet pea flowers is also due to flavonoids. However, flavonoids contained in sweet pea flowers are paler ones of which chromophores are kaempferol, quercetin or myricetin (Sakata and Arizumi, 1983). Likewise, it was thought that the light-yellow coloration of Eustoma flowers is due to flavonoids of which chromophores are quercetin and its derivative isorhamnetin (Markham and Ofman, 1993). However, we showed that carotenoids are the pigments responsible for the yellow coloration of Eustoma flowers (Nakayama et al., 2006). This raised the possibility that the yellow coloration of sweet pea flowers may also be due to carotenoids. If this is correct, it should be possible to breed deep-yellow flower cultivars.
Observation of sweet pea flowers under ultraviolet light and blue light in dark conditions suggested that the responsible pigments for the light-yellow coloration are not flavonoids, but carotenoids. We proceeded with analyses to confirm this hypothesis. Information on genes regulating the target trait is essential to conduct efficient breeding plans. Here, we investigated the heredity of yellow coloration in flowers of progenies obtained by crossing ‘Artemis’ and a white cultivar ‘Diana White’ or a yellow cultivar ‘Stella’.
The sweet pea cultivars used in this study were as follows: ‘Artemis’, ‘Jilly’, and ‘Stella’ (light-yellow flower cultivars); ‘Easter Parade’, ‘Diana White’, ‘Robe Decollete’, ‘Sweet Snow’, and ‘Com-Pur W’ (white flower cultivars); ‘Candyman’ (salmon pink striped variegated flower cultivar), ‘Susie’(salmon pink flower cultivar), ‘Geranium Pink’(dark salmon pink flower cultivar), ‘Red Ensign’ (reddish orange flower cultivar), ‘Annie Good’(purplish pink flower cultivar), and ‘Lord Nelson’ (dark reddish purple flower cultivar). Cold treatment at 2°C in dark conditions was performed on water-absorbed seeds of the spring-flowering cultivar ‘Susie’ and ‘Stella’ for four weeks, and the summer-flowering cultivars: ‘Annie Good’, ‘Candyman’, ‘Geranium Pink’, ‘Jilly’, ‘Lord Nelson’, and ‘Red Ensign’ for six weeks, respectively. This treatment was not performed on seeds of the winter-flowering cultivars: ‘Artemis’, ‘Easter Parade’, ‘Diana White’, Robe Decollete’, ‘Sweet Snow’, or ‘Com-Pur W’. These seeds were planted on planting beds (0.8 m width, two row planting with a 0.6 m row pitch, 0.1 m planting distance) or pots (0.18 m diameter) containing medium mixing loamy soil: leaf mold: peat = 2:1:1 (volume ratio). These were grown in a greenhouse at Kanagawa Agricultural Technology Center (Hiratsuka, Kanagawa, Japan) under natural light with controlled temperature at 5–30°C by heating or ventilation.
F1 plants were obtained by crossing ‘Artemis’ and ‘Diana White’. F2 plants were obtained by selfing F1 plants. The other F1 plants were obtained by crossing ‘Artemis’ and ‘Stella’. Seeds of these F1 and F2 plants were planted without cold treatment and grown as described above. The number of plants segregated by flower coloration properties were counted.
UV and blue light observation of flowersPetals of light-yellow and white cultivars were observed under ultraviolet light and blue light (Nakayama et al., 2006). Samples were placed in a dark chamber (CSN-15 AC/AC; Cosmo Bio Co. Ltd., Japan), and irradiated by ultraviolet light (365 nm). Samples were placed in a dark room and irradiated by blue light (around 470 nm) using an LED panel (LED-B; Tokyo Rikakikai Co. Ltd., Japan).
Extraction of yellow pigmentStandard and wing petals of ‘Artemis’, devoid of basal green parts, were frozen with liquid nitrogen and stored at −30°C. Small amounts of MgCO3 were added to the petal samples to maintain weak basic conditions to prevent degradation of carotenoids, and samples were ground using a mortar and pestle in EtOH to extract yellow pigments. The yellow pigments were extracted again with EtOH, and then with acetone three times. The extracts were combined and evaporated to dryness. The dried residue was dissolved in EtOAc and fractioned with distilled water three times. The EtOAc fraction was dried over Na2SO4 and evaporated to dryness.
Similar procedures were carried out with petals of cyanic flower cultivars of ‘Annie Good’, ‘Candyman’, ‘Geranium Pink’, ‘Jilly’, ‘Lord Nelson’, ‘Susie’, and ‘Red Ensign’ (Fig. 3). Obtained dry residues were dissolved with EtOAc containing 20% n-hexane. The solution was passed through a silica gel column to remove undissolved impurities and evaporated to dryness.
Spectroscopic analysisThe extracted yellow pigments were dissolved in EtOAc and their absorption spectra at 350–700 nm were analyzed using a UV-2450 spectrophotometer (Shimazu Co., Japan). Carotenoid concentrations were calculated as lutein equivalents based on absorbance at 446 nm.
HPLC analysis of carotenoidsA portion of the yellow pigments extracted from ‘Artemis’ was dissolved in 5% KOH/MeOH and hydrolyzed at room temperature for 3 h. After adding saturated salt water, the yellow pigments were extracted with EtOEt and then washed with water two times. The EtOEt fraction was dried over Na2SO4 and evaporated to dryness. Hydrolyzed and non-hydrolyzed pigments were dissolved in EtOAc and analyzed by HPLC using a Cosmosil 5C18 MS-II column (4.6 × 250 mm; Nacalai Co., Japan) according to the elution and recording methods of Nakayama et al. (2006). The carotenoids were identified by comparing retention time and absorbance spectra with commercial standard samples.
Extraction and HPLC analysis of flavonoidsStandard and wing petals of ‘Artemis’ were frozen in liquid nitrogen and stored at −30°C. Samples were ground in 10% aq. AcOH to extract flavonoids. Flavonoids were extracted again with 10% aq. AcOH. The extracts were combined. Similar procedures were carried out with petals of white flower cultivars ‘Easter Parade’, ‘Diana White’, and ‘Robe Decollete’. The extracted flavonoids were analyzed by HPLC with an Agilent HPLC 1100 system using an Inertsil ODS-2 column (4.6 × 250 mm; GL Science Inc., Japan). Elution of solvent A (1.5% phosphoric acid) and solvent B (1.5% phosphoric acid, 20% acetic acid, and 25% MeCN) was performed with a 20–80% linear gradient of solvent B in solvent A for 0–40 min with a flow rate of 0.8 mL·min−1 at 40°C. The absorbance spectra at 300–600 nm were recorded using the photodiode array detector in the HPLC system. The flavonoids were putatively identified based on absorbance spectra characteristics.
While flavonoids strongly absorb ultraviolet light, carotenoids strongly absorb blue light. The petals of light-yellow flowers of ‘Artemis’ and white flowers of ‘Diana White’, ’Robe Decollete’, ‘Easter Parade’, ‘Sweet Snow’, and ‘Com-Pur W’ showed similar dark images under ultraviolet light (Fig. 1B). The petals of ‘Artemis’ had darker image than ‘Diana White’ under blue light (Fig. 1C).
Analyses of carotenoids in ‘Artemis’The yellow pigments extracted from ‘Artemis’ petals strongly absorbed light between 350 and 525 nm (Fig. 2A). Three absorption peaks (λmax) were detected at 421 nm, 446 nm, and 474 nm. The HPLC chromatogram of the hydrolyzed extract from ‘Artemis’ petals had five major peaks of absorption at 440 nm (Fig. 2B). The peaks were assigned to lutein, zeaxanthin and β-carotene based on a comparison with the retention time and absorption peaks of standard samples (Table 1). Based on the peak area of absorption at 440 nm, lutein was the main carotenoid pigment, representing 58% of total carotenoids, followed by zeaxanthin and β-carotene, representing 21% and 6% of total carotenoids, respectively. The HPLC chromatogram of the crude extract did not have peaks of these carotenoids as major ones, and instead had many other peaks which could not be detected in the hydrolyzed extract (Fig. 2C).
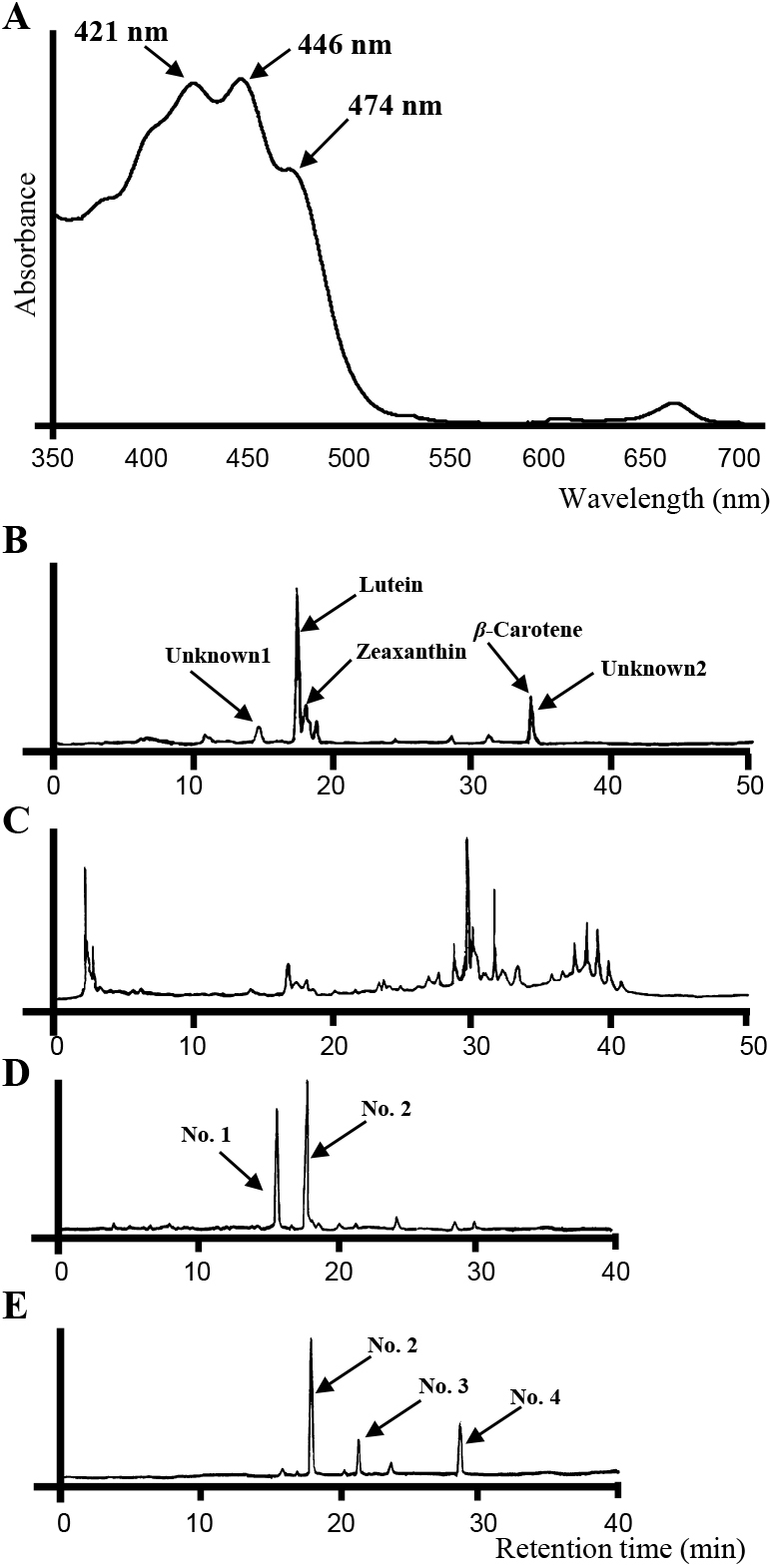
Analyses of yellow pigments contained in petals of ‘Artemis’. (A) Absorption spectrum of the yellow pigments. (B) HPLC chromatograms of hydrolyzed and (C) non-hydrolyzed carotenoids. (D) HPLC chromatograms of flavonoids of ‘Artemis’ and (E) ‘Diana White’. No. 1-4 indicate flavonoids identical to those presented in Table 2.
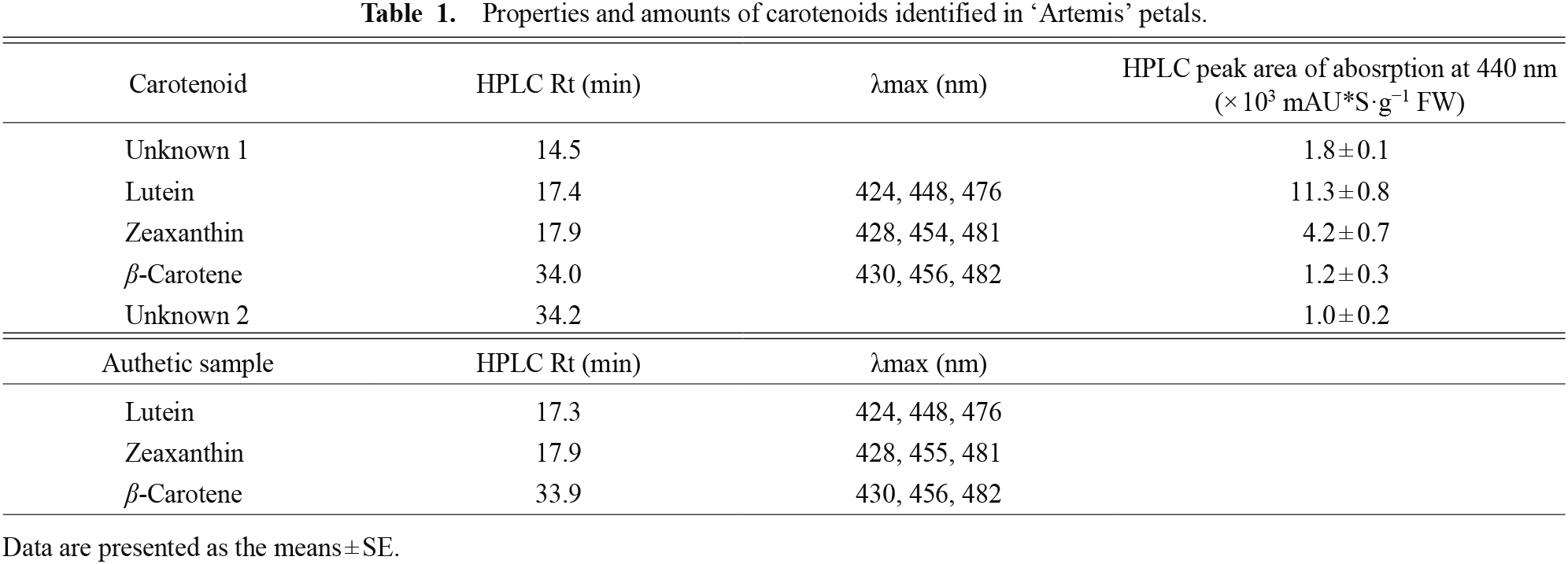
Properties and amounts of carotenoids identified in ‘Artemis’ petals.
HPLC chromatograms of extracts from ‘Artemis’ and white flowers of ‘Diana White’, ‘Easter Parade’, and ’Robe Decollete’ showed several peaks when monitored by absorption at 360 nm (Fig. 2D, E). The main peaks were putatively assigned to flavonoids of which chromophores were kaempferol and quercetin derivatives based on the absorption spectra characteristics (Table 2). The relative amount of these flavonoids in each cultivar was calculated based on peak area of absorption at 360 nm. ‘Artemis’ contained similar to, or lower levels than, total flavonoids in the white cultivars.
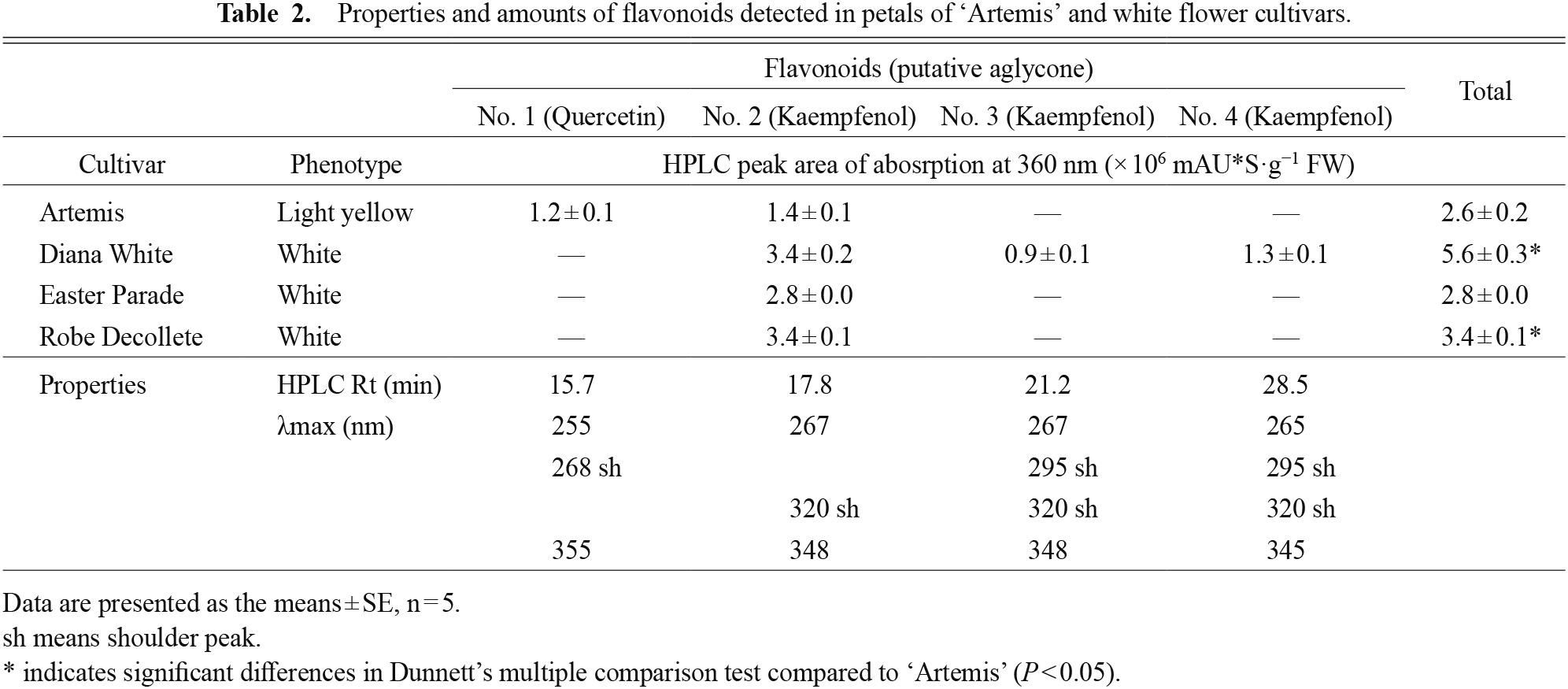
Properties and amounts of flavonoids detected in petals of ‘Artemis’ and white flower cultivars.
Spectroscopic analyses were performed to calculate total amounts of carotenoid contained in petals of yellow and cyanic cultivars as lutein equivalents (Fig. 3; Table 3). The total amount of carotenoids in ‘Artemis’ was 7.47 nmol·g−1 FW. Crude extracts of another light-yellow cultivar ‘Jilly’, a salmon pink striped variegated flower cultivar ‘Candyman’, a deep salmon pink cultivar ‘Geranium Pink’, a reddish orange cultivar ‘Red Ensign’, and a salmon pink cultivar ‘Susie’ showed characteristic absorption spectra of carotenoids, but a purplish pink cultivar ‘Annie Good’ and a dark purplish red cultivar ‘Lord Nelson’ did not (Table 3). The total amount of carotenoids of ‘Red Ensign’ was similar to that of ‘Artemis’, and those of ‘Jilly’ and ‘Geranium Pink’ were 0.1 to 0.5 times lower than that of ‘Artemis’, while those of ‘Candyman’ and ‘Susie’ were about 1.4 and 2.0 times greater than that of ‘Artemis’, respectively.

Cultivars used for analyses of carotenoid contents. 1, ‘Annie Good’; 2, ‘Candyman’; 3, ‘Geranium Pink’; 4, ‘Jilly’; 5, ‘Lord Nelson’; 6, ‘Red Ensign’; 7, ‘Susie’.
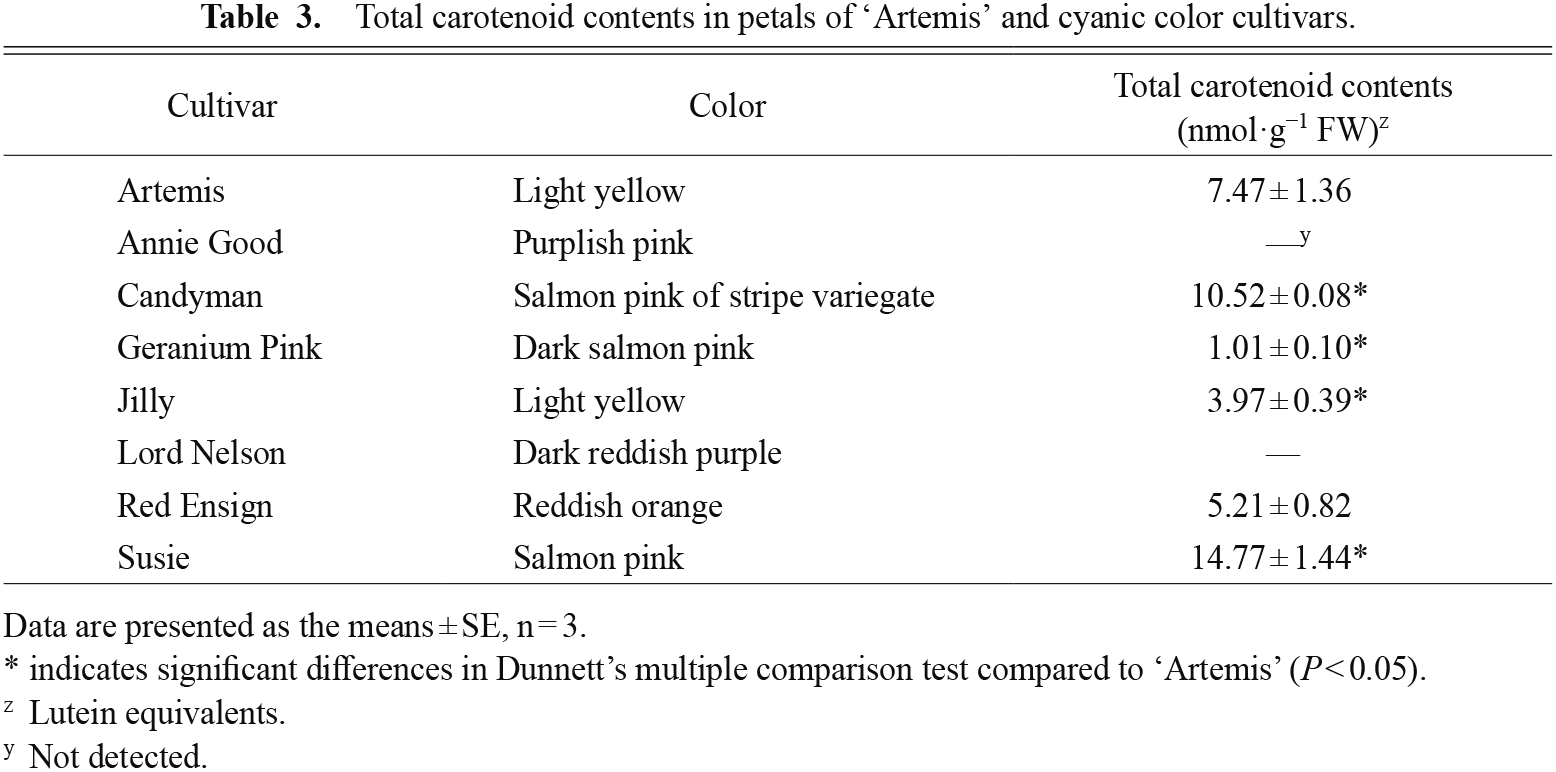
Total carotenoid contents in petals of ‘Artemis’ and cyanic color cultivars.
Flowers of the F1 and F2 plants were classified into four coloration phenotypes: white, yellow, red and a combination of yellow and red. Because cyanic coloration does not appear in the basal part of the flower, expression of yellow coloration was evaluated based on coloration of the basal part (Fig. 4). The yellow and red type combination, which has red petals with a yellow basal petal, is hereafter described as yellow-red. Although the red coloration of petals varied in depth, the classification was performed regardless of the depth.
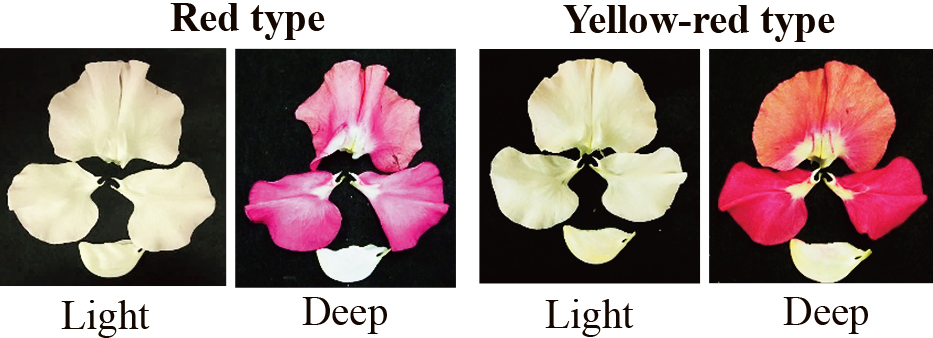
Red and yellow-red type flowers of F1 and F2 plants obtained by crossing between ‘Artemis’ and ‘Diana White’. Both have light and deep cyanic color types. Images were artificially adjusted to match those of original images.
All three F1 plants obtained by crossing ‘Artemis’ and ‘Diana White’ were the red type. Among the 33 plants in the F2 plants, 14 were white type, 2 were yellow type, 10 were red type, and 7 were yellow-red type (Tables 4 and 5). All 18 F1 plants obtained by crossing ‘Artemis’ and ‘Stella’ were the yellow-red type.
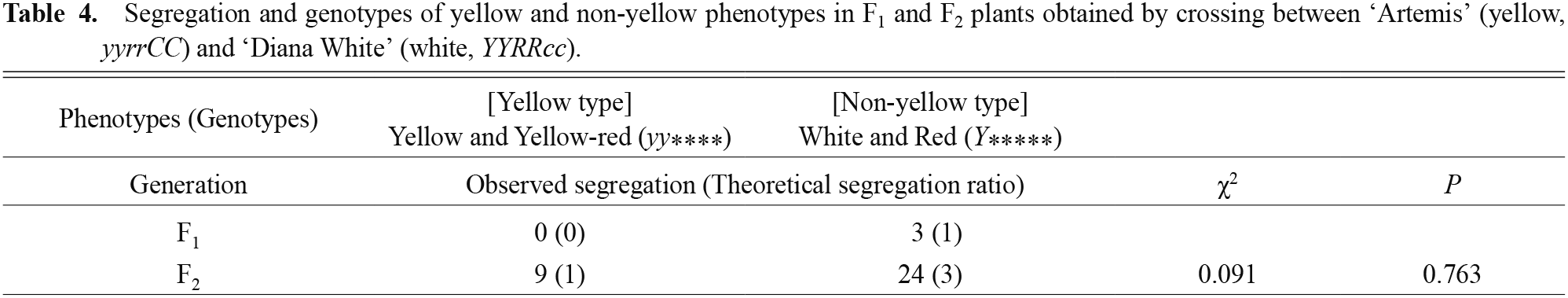
Segregation and genotypes of yellow and non-yellow phenotypes in F1 and F2 plants obtained by crossing between ‘Artemis’ (yellow, yyrrCC) and ‘Diana White’ (white, YYRRcc).

Segregation and genotypes of acyanic and cyanic color phenotypes in F1 and F2 plants obtained by crossing between ‘Artemis’ (yellow, yyrrCC) and ‘Diana White’ (white, YYRRcc).
Yellow coloration is expressed by absorption of blue light between 400 nm and 500 nm. Flavonoids absorb blue light a little, and ultraviolet light strongly, around 360 nm, resulting in light-yellow coloration. Conversely, carotenoids absorb blue light strongly, around 440 nm, and ultraviolet light a little, resulting in deep yellow coloration. Therefore, the amounts of carotenoids and flavonoids can be roughly compared among cultivars based on the darkness of the petal image under blue and UV light, respectively (Nakayama et al., 2006). A light-yellow ‘Artemis’ flower produced a darker image than white flowers of other cultivars under blue light, but did not under UV light (Fig. 1), suggesting that carotenoids are possibly responsible for the light-yellow coloration. We further performed spectroscopic analysis of the yellow pigments extracted from ‘Artemis’ flowers and the absorption spectrum was typical of carotenoids (Fig. 2A). The total carotenoid amount was a similar level to that of the light-yellow flower of Eustoma (Table 3) (Nakayama et al., 2006). We confirmed that total flavonoid amount of ‘Artemis’ flowers was lower than that of white flowers (Table 2). We conclude that the pigments responsible for the light-yellow coloration of sweet pea flowers are not flavonoids, but carotenoids. Because lutein is a major yellow pigment (Table 1), like marigold and sunflower (Ohmiya, 2011), we expect to be able to generate sweet pea cultivars with deep-yellow flowers.
In order to obtain information on the heredity of the yellow coloration, we crossed ‘Artemis’ with a white cultivar, ‘Diana White’. The yellow trait is recessive because yellow coloration did not appear in the F1 plants (Table 4). If the yellow coloration is regulated by a single recessive gene (y gene), the theoretical segregation ratio of yellow type: non-yellow type in the F2 plants is 1:3. Observed segregation of the yellow type (whole yellow type and yellow-red type): non-yellow type (white type and red type) was 9:24. No significant difference between the observed and theoretical segregation ratios was found by using the chi square test (χ2 = 0.091, P = 0.763), thus validating our hypothesis that the yellow trait is regulated by a single recessive gene (y gene).
Punnett (1923) reported that in sweet pea, complementary genes of R-white (r) and C-white (c) are involved in cyanic coloration. Biosynthesis of anthocyanins is inhibited when either locus is a homologous recessive gene, generating acyanic color flowers. Because the genotype of ‘Diana White’ is RRcc (Yagishita et al., 2018) and all F1 plants obtained by crossing ‘Artemis’ and ‘Diana White’ were of the cyanic type (Table 5), the genotype of ‘Artemis’ must be rrCC. In this case, the theoretical segregation ratio of the acyanic type: cyanic type in the F2 plants is 7:9. Observed segregation of the acyanic type (white type and yellow type): cyanic type (red type and yellow-red type) was 16:17. No significant difference between the observed and theoretical segregation ratios was found by using the chi square test (χ2 = 0.301, P = 0.583), thus validating our hypothesis that the genotype of ‘Artemis’ is rrCC.
Because all F1 plants obtained by crossings between ‘Artemis’ and another light-yellow cultivar, ‘Stella’, were the yellow-red type, the genotype of ‘Stella’ must be RRcc. If the yellow phenotype of ‘Stella’ is regulated by the same y gene, the Y, R and C loci may be independent. If so, theoretical phenotypes, genotypes and their segregation ratios for F2 plants using ‘Artemis’, for which the genotype is yyrrCC, as one parent are shown in Table 6. As a result, carotenoid accumulation and anthocyanin accumulation can be combined or separated. In fact, among cyanic cultivars, ‘Candyman’ and ‘Susie’ contained higher amounts of carotenoids than ‘Artemis’ (Table 3). Therefore, these cultivars are promising parents to generate more intense yellow cultivars.
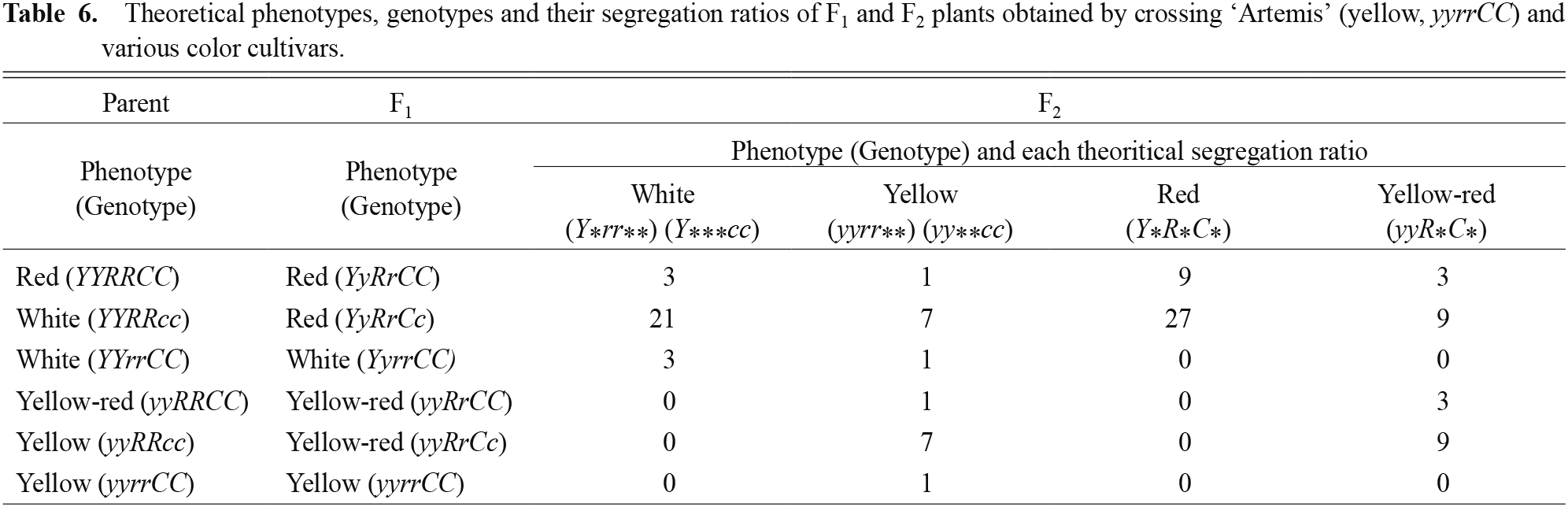
Theoretical phenotypes, genotypes and their segregation ratios of F1 and F2 plants obtained by crossing ‘Artemis’ (yellow, yyrrCC) and various color cultivars.
Carotenoids contained in petals are generally acylated with fatty acids; inhibition of acylation decreases accumulation of carotenoids (Ohmiya and Yamamizo, 2010). Drastic differences in HPLC chromatograms between hydrolyzed and non-hydrolyzed carotenoids indicate that a large portion of carotenoids contained in sweet pea petals are acylated (Fig. 2B, C), hinting that the y may encode an enzyme involved in acylation. However, this is unlikely because such a gene must be a dominant trait. The Y may have a role in carotenoid degradation like CmCCD4a in Chrysanthemum flowers, in which expression of the yellow coloration trait is recessive (Ohmiya et al., 2006).
ConclusionWe identified carotenoids as the responsible pigments for the yellow coloration of sweet pea petals. Accumulation of carotenoids is regulated by a single recessive gene and carotenoid accumulation and anthocyanin accumulation can be combined or separated. Among cyanic color cultivars, we found some that contained higher amounts of carotenoids.