2023 年 92 巻 4 号 p. 431-438
2023 年 92 巻 4 号 p. 431-438
Prunus fruit trees of the Rosaceae family exhibit S-RNase-based gametophytic self-incompatibility (GSI), which enables pistils to reject self-pollen by suppressing pollen tube elongation. In other plant species with S-RNase-based GSI, it has been shown that this suppression consists of two steps: first, slowing down of pollen tube elongation in the middle part of the style and second, complete arrest involving programmed cell death. To characterize the suppression pattern of incompatible pollen tubes in Prunus, we observed pollen tube elongation of ‘Satonishiki’ sweet cherry (Prunus avium) in ‘Satonishiki’ and ‘Rainier’ pistils on agar plates and ‘Satonishiki’ and ‘Rainier’ pollen tube growth in pistils on ‘Satonishiki’ cut branches. Incompatible selfed pollen tubes delayed penetration into the stigma in both experiments. Observation of pollen tubes in pistils on agar plates was difficult 24 h after pollination (HAP) due to wilting of the styles, while observing them on cut branches was possible up to 72 HAP. In the pistils on cut branches, ‘Satonishiki’ pollen tubes barely elongated in self pistils from 32 to 48 HAP when compatible ‘Rainier’ pollen tubes reached the base of a style, but resumed growth after 48 HAP and reached the base of the style. An RNase activity staining indicated that S-RNase was inactive 48 HAP. Finally, we observed pollen tube elongation in the style-grafted pistils on the cut branches. ‘Satonishiki’ pollen tube elongation was accelerated when the upper one-third of the self-pollinated styles was grafted onto compatible ‘Taishonishiki’ pistils. These results indicated that the suppression of incompatible pollen tube elongation in P. avium was consistently reversible. We discuss the suppression mechanism of incompatible pollen tube growth and the possibility of artificial control of Prunus self-incompatibility.
Most of the fruit tree species in Prunus exhibit self-incompatibility (SI), a genetic mechanism that enables pistils to recognize cognate pollen and reject its fertilization (de Nettancourt, 2001). SI is an obstacle to efficient fruit production and cross breeding in Prunus fruit trees. The SI mechanism of Prunus is classified as S-RNase-based gametophytic SI (GSI), in which S-RNase and F-box proteins are the pistil and pollen determinant, respectively. S-RNase is secreted into the transmitting tract of pistils and acts as a toxin to incompatible pollen tubes (Anderson et al., 1989; Huang et al., 1994). S-RNase-based GSI is assumed to be a mechanism ancestral to dicots, since it is distributed in Solanaceae, Plantaginaceae, Rosaceae, Rubiaceae, and Rutaceae. Recent genomic studies have revealed that genes related to S-RNase-based GSI have undergone lineage-specific evolution (Aguiar et al., 2015; Akagi et al., 2016; Anderson et al., 1986; Igic and Kohn, 2001; Liang et al., 2020; Lv et al., 2022; Morimoto et al., 2015; Nowak et al., 2011; Sassa et al., 1992; Xue et al., 1996). S-RNase-based GSIs in the Rosaceae family have evolved in an interesting manner. The S-RNase-based GSI of Maleae of the Rosaceae family is a non-self-recognition system similar to that of Solanaceae and Plantaginaceae, in which the pollen determinant F-box proteins recognize and detoxify S-RNases other than the cognate ones. However, that of Prunus of the same family is a self-recognition system in which the pollen determinant F-box protein recognizes and triggers the cytotoxicity of cognate S-RNase (Kakui et al., 2011; Kubo et al., 2010; Matsumoto and Tao, 2016; Qiao et al., 2004; Sijacic et al., 2004). The commonality and specificity in incompatible reactions is still unclear between Prunus and the other species. Understanding the Prunus incompatible reaction would be useful to develop artificial control of Prunus SI, which would contribute to stable fruit production and/or facilitate breeding programs.
The mechanism of incompatible reactions within pollinated pistils has been studied mainly in Solanaceae. It is thought that although S-RNase uptake was sequestered in vacuoles in incompatible pollen tubes, S-RNase leaked into the cytoplasm due to vacuolar disruption and degraded RNA, thus preventing incompatible pollen tube elongation, based on in vitro experiments and confocal microscopic observation in Nicotiana alata (Goldraij et al., 2006; McClure et al., 1990). Subsequent detailed microscopic observations revealed that disorganization of the F-actin cable in incompatible pollen tubes preceded vacuolar disruption, which is concomitant with the slowing of incompatible pollen tube elongation (Roldán et al., 2012). In this study, incompatible pollen tubes showed slowed elongation 1 d after pollination (DAP) and this ceased after reaching the lower one-third of a style at 5 DAP. In Solanum chacoense, incompatible pollen tube elongation began to slow down at 12 h after pollination (HAP) and stopped at the upper one-third at 18 HAP, whereas RNA degradation in incompatible pollen tubes was not observed at 12 HAP, but rather at 24 HAP (Liu et al., 2012). Vacuolar disruption is often associated with programmed cell death. In Petunia hybrida, the hallmarks of programmed cell death are observed in incompatible pollen tubes (Kovaleva et al., 2020). Taken together, these results suggest that the elongation of incompatible pollen tubes is suppressed in two steps: 1) slowing down of elongation involved in the disruption of F-actin cables and 2) complete arrest involved in RNA degradation and programmed cell death associated with vacuolar disruption. Although the molecular mechanism underlying these steps is still under study, it has also been reported that S-RNase binds to the actin bundle via eEF1A (Soulard et al., 2014) and that the actin ring in the V-shaped zone of incompatible pollen tubes disappeared and membrane aggregates accumulated prior to the collapse of the F-actin cables (Roldán et al., 2015).
Incompatible pollen tubes in the Japanese pear Pyrus pyrifolia in Maleae of the Rosaceae family were reported to elongate and stop similar to those of Solanaceae. Although there is no difference in pollen tube elongation between compatible and incompatible pollen tubes, incompatible pollen tubes begin to slow down and cease elongation at the base of the style (Hiratsuka and Tezuka, 1980). Although it is not clear whether the process of suppression of incompatible pollen tube elongation in vivo is similar to that in Solanaceae, a two-step suppression of incompatible pollen tube elongation has been observed in vitro, where F-actin bundle pollen tubes are disrupted by S-RNase treatment and programmed cell death subsequently occurs (Chen et al., 2018; Liu et al., 2007; Wang et al., 2009). Unlike in Solanaceae, the S-RNase of the Japanese pear has been shown to bind actin directly (Chen et al., 2018).
However, the growth patterns of incompatible pollen tubes in Prunus spp. have not yet been studied in detail. Although experimental replications were lacking in a study by Lewis (1942), incompatible pollen tubes were shorter than compatible ones at first observation at 24 HAP, and thereafter elongation was significantly slower. In this study, we made detailed observations of self and nonself pollen tube growth of sweet cherry (P. avium) in intact and style-grafted pistils.
Mature trees of P. avium ‘Satonishiki’ (S3S6) planted in the experimental orchard of Yamagata University, and pot-grown trees of ‘Rainier’ (S1S4) and ‘Taishonishiki’ (S1S4) were used in the present study (Takahashi et al., 2015). Anthers were collected from flower buds immediately before anthesis, dehisced by overnight incubation with silica gel, and the pollen obtained was stored with silica gel at −80°C. In the following year, the collected pollen was used for the pollination tests.
Observation of pollen tube elongation in pistils on agar platesFlower buds of ‘Satonishiki’ and ‘Rainier’ were collected immediately after flowering and emasculated. The peduncle ends were removed using a razor. Excised pistils were inserted into 1% agar prepared in a petri dish, and 10–16 h later, ‘Satonishiki’ pollen was pollinated and incubated at 20°C. At 3, 6, 9, 16, 24, and 32 HAP, pollinated pistils were fixed overnight with Carnoy fixative solution (chloroform: 95% ethanol: glacial acetic acid = 1:3:1) and then replaced with 99% ethanol and kept until observation. For the staining of pollen tubes in pistils, samples were softened overnight in 10 N NaOH and stained for callose with 0.1% aniline blue overnight. The prepared samples were placed on glass slides and lightly squashed under a cover slip. The pollen tubes within the pistils were observed under a fluorescence microscope (ECLIPSE Ni series; Nikon Instec, Inc., Tokyo, Japan) to measure the length of the longest pollen tube. At each time point, 10–13 pollinated pistils were examined for both incompatible and compatible pollination, and differences in means were tested using the Student’s t-test.
Observation of pollen tube elongation in pistils on cut branchesTwo- to three-year-old branches of ‘Satonishiki’ (S3S6) and ‘Taishonishiki’ (S1S4) were used. The cut branches were placed in tap water in a beaker and kept in a lab (20°C). The base of the cut branches was cut back by approximately 1 cm, and tap water in the beaker was replaced each day. The flower buds on the ‘Satonishiki’ cut branches that were expected to open within 6 h were emasculated, whereas the others were removed. They were pollinated with ‘Satonishiki’ pollen (incompatible pollen) or ‘Rainier’ pollen (compatible pollen) 24 h after emasculation. Compatible and incompatible pollinated pistils were sampled 8, 16, 24, 32, 48, and 72 HAP. For style-grafting, one-third of the stigmatic ends of styles of compatible-pollinated pistils 8 HAP and those of incompatible-pollinated pistils 16 HAP were excised using a razor and grafted on the ‘Satonishiki’ or ‘Taishonishiki’ pistils from which the equivalent area was removed. Rootstock-pistils were prepared and emasculated simultaneously with pistils for scion-styles. A drop of 10% sucrose was applied to the cut surface to reduce browning, and a silicon tube with an inner diameter of 0.5 mm was used to support scion-styles (Fig. 1). After style-grafting, the cut branches were placed in a handmade chamber of a polystyrene bag together with a humidifier to prevent wilting of the scion-styles. Style-grafted pistils were sampled 16 h after grafting, fixed, stained, and observed as described above. Eight pistils were examined at each time point for both compatible and incompatible pollination, while six style-grafted pistils were examined for each style-grafting experiment, and differences in means were tested using the Student’s t-test.

Style-grafted pistils on ‘Satonishiki’ cut branches (left panel) and a sample 16 h after style-grafting (right panel). A scion-style was supported by a silicon tube with an inner diameter of 0.5 mm.
Styles were collected 1–3 days after anthesis (DAA) from prepared cut branches of ‘Satonishiki’ as described above and cut into the following parts: 1 mm at the tip of the styles, including the stigma, and the upper and lower halves of the left styles, which were then frozen in liquid nitrogen and stored at −80°C until use. Each part of the style was homogenized using a pestle in the extraction buffer [50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 5 mM 2-mercaptoethanol, 0.2% (v/v) Triton X-100, and 1 × Complete Protease Inhibitor Cocktail (Roche, Basel, Switzerland)]. Recombinant PavS3-RNase, which was tagged with DDDDK at the N-terminus, was expressed using the silkworm-baculovirus system (Sysmex, Kobe, Japan) and purified using a DDDDK-tagged protein purification kit (MBL & Biological Laboratories, Nagoya, Japan), as described by Matsumoto and Tao (2019). Protein concentration was determined using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA) with bovine serum albumin as the standard.
For immunoblotting, protein samples were separated on 12% SDS-polyacrylamide gels. Proteins in the gel were blotted onto polyvinylidene difluoridemembranes (Millipore, Billerica, MA, USA), and antiserum against PavS3-RNase was used as the primary antibody, as previously described by Matsumoto and Tao (2012). Immune complex signals were detected using an ECL Advance Western Blotting Detection Kit (Cytiva, Marlborough, MA, USA) and ImageQuant LAS500 system (Cytiva). PavS3-RNase amounts in the samples were estimated using a standard curve based on the signal intensity of the dilution series of the recombinant PavS3-RNase.
For RNase-activity staining, protein samples were separated on 12% non-reducing SDS-polyacrylamide gels. The gels were immersed in a series of solutions in the following order: isopropanol for 10 min twice, 10 mM tris-HCl (pH 7.4) for 10 min twice, 0.4% RNA solution (w/v; RNA from torula yeast, type IV; Sigma-Aldrich, Burlington, MA, USA) in 10 mM Tris-HCl (pH 7.4) for 20 min at 37°C, 100 mM Tris-HCl (pH 7.4) for 3 min at 50°C, and 2% toluidine blue solution. The stained gels were washed with 10 mM Tris-HCl (pH 7.4) until RNase bands appeared.
Most of the pistils began to turn brown and wilt 50 h after being placed on agar plates. ‘Satonishiki’ pollen pollinated to ‘Rainier’ pistils (compatible pollination) germinated and penetrated the stigma in 50% of the samples at 3 HAP (5/10) and in all samples at 6 HAP (13/13). In contrast, ‘Satonishiki’ pollen pollinated to ‘Satonishiki’ pistils (incompatible pollination) did not penetrate into the stigma in any samples at 3 HAP (0/11) and penetrated 50% of the samples at 6 HAP (6/12), in 55% at 9 HAP (6/11), and in all at 16 HAP (12/12). In both the ‘Rainier’ and ‘Satonishiki’ pistils, the rate of pollen tube elongation drastically increased after passing through the top 1 mm of the style, including the stigma. Pollen tubes continued to grow until 24 HAP, but significantly slowed after that (Fig. 2). This indicated that the pollen tube could not elongate in pistils on agar plates after 24 HAP due to pistil senescence, but not because of incompatible reactions. At 32 HAP, ‘Satonishiki’ pollen tubes reached approximately 80% of the total length of the ‘Rainier’ styles (8.13 mm/10.06 mm) and approximately 60% of the ‘Satonishiki’ styles (5.55 mm/8.97 mm) (Fig. 2).
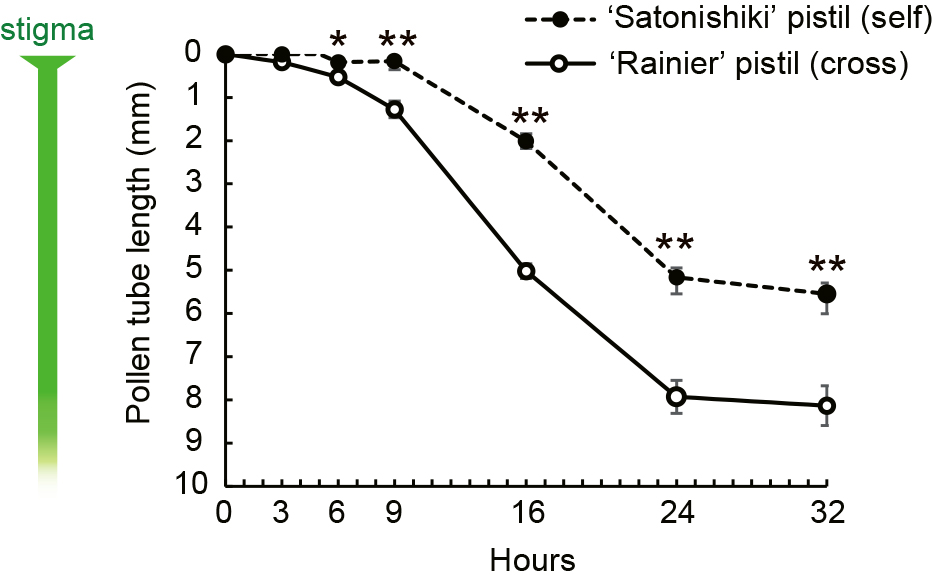
‘Satonishiki’ (S3S6) pollen tube growth in the compatible (‘Rainier’ (S1S4)) and incompatible (‘Satonishiki’ (S3S6)) pistils on 1% (w/v) agar plates. The data show the mean length of the longest pollen tube in the styles (n = 10–13). The mean ± SE of ‘Rainier’ and ‘Satonishiki’ style length were 10.06 ± 0.10 mm (n = 75) and 8.97 ± 0.07 mm (n = 72), respectively. Single and double asterisks indicate significant differences between the compatible and incompatible pollen tube length at P < 0.05 and P < 0.01, respectively.
The rates at which ‘Satonishiki’ pollen tubes elongated steadily after passing through the stigma were 393 μm/h in self styles (16–24 HAP) and 443 μm·h−1 in ‘Rainier’ styles (9–24 HAP).
Growth patterns of the incompatible pollen tubes in pistils on cut branchesMost of the pistils emasculated on the cut branches began to turn brown at 3 DAA and abscised at 4 DAA. In all the observed samples at 8 HAP, ‘Rainier’ pollen (compatible pollen) penetrated through the stigma of ‘Satonishiki’ pistils, whereas ‘Satonishiki’ pollen (incompatible pollen) elongated within their stigma (Fig. 3). The ‘Rainier’ pollen tubes continued to elongate steadily, reaching the base of the styles by 48 HAP (Figs. 3 and 4). The ‘Satonishiki’ pollen tubes also continued to elongate steadily until 32 HAP, but ceased to grow at approximately 70% of the total length of styles (6.22 mm/9.02 mm) until 48 HAP. However, the ‘Satonishiki’ pollen tubes resumed elongation after 48 HAP and eventually reached the base of the styles by 72 HAP (Figs. 3 and 4).

Compatible (‘Rainier’ (S1S4)) and incompatible (‘Satonishiki’ (S3S6)) pollen tube growth in ‘Satonishiki’ (S3S6) pistils on cut branches. The data show the mean length of the longest pollen tube in styles (n = 8). The mean ± SE of the ‘Satonishiki’ style length was 9.10 ± 0.06 mm (n = 96). Single and double asterisks indicate significant differences between the compatible and incompatible pollen tube length at P < 0.05 and P < 0.01, respectively.
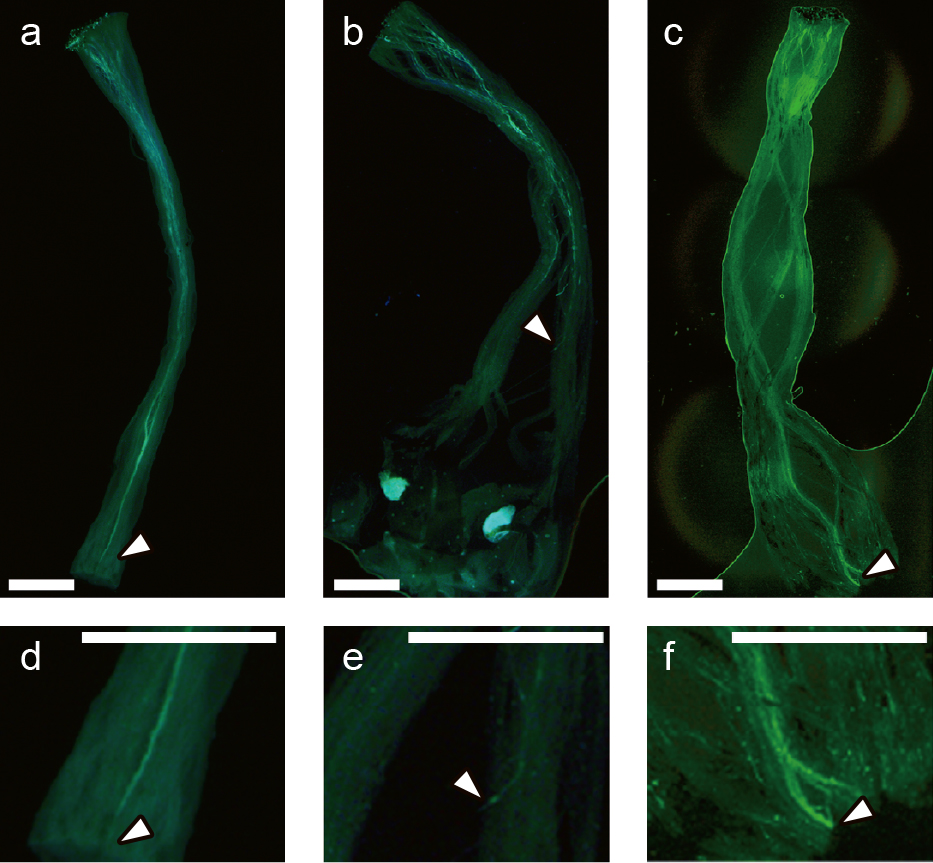
Aniline blue staining of pollen tubes in the ‘Satonishiki’ (S3S6) pistils on cut branches. (a) Compatible (‘Rainier’ (S1S4)) pollen tubes in the style at 32 h after pollination. (b) Incompatible (‘Satonishiki’ (S3S6)) pollen tubes in the style at 32 h after pollination. (c) Incompatible pollen tubes in the style at 72 h after pollination. (d–f) Close-up of the longest pollen tubes in a–c, respectively. Arrowheads indicate the tip of the longest pollen tube. Scale bars are 1 mm.
The rate at which ‘Satonishiki’ pollen tubes elongated steadily after passing through stigma was 220 μm·h−1 in self styles (16–32 HAP), while that of ‘Rainier’ pollen tubes was 237 μm·h−1 (8–32 HAP) in ‘Satonishiki’ styles.
Changes in the amount and activity of PavS3-RNase in styles after anthesisThe amount of PavS3-RNase in the ‘Satonishiki’ style was determined by an immunoblotting assay done for three parts: 1 mm at the tip of styles, including the stigma, and the upper and lower halves of the left styles (Fig. 5). The upper and lower halves were approximately from 1 mm to 5 mm and from 5 mm to 9 mm from the stigma, respectively. Silkworm-expressed recombinant DDDDK-tagged PavS3-RNase was used as the standard. The amount of PavS3-RNase in each part of the ‘Satonishiki’ style was calculated as the recombinant S-RNase equivalent, although the molecular weight of silkworm-expressed recombinant S-RNase was a little bigger than the innate one due to the presence of the DDDDK-tag and linker peptide and multiple N-glycosylation sites, which are thought to be glycolyzed differently in plants and insects (Strasser, 2016). The stigma and the upper halves of styles contained an abundant amount of PavS3-RNase, whereas the lower halves of the styles did not (20.59–26.11 ng/pistil, 41.49–53.19 ng/pistil, and 0.56–1.08 ng/pistil, respectively; Table 1). PavS3-RNase protein was significantly decreased in the lower half of the styles from 2 DAA, but not in the other parts.

Semi-quantification of PavS3-RNase in the stigmas and styles of ‘Satonishiki’ (S3S6) 1–3 days after anthesis (DAA) by protein blot analysis. Crude extracts in each lane were of 0.4, 0.3, and 5 pistils for the stigma, and the upper and lower halves of the style, respectively. Purified recombinant DDDDK-tagged PavS3-RNase were loaded alongside as a standard.
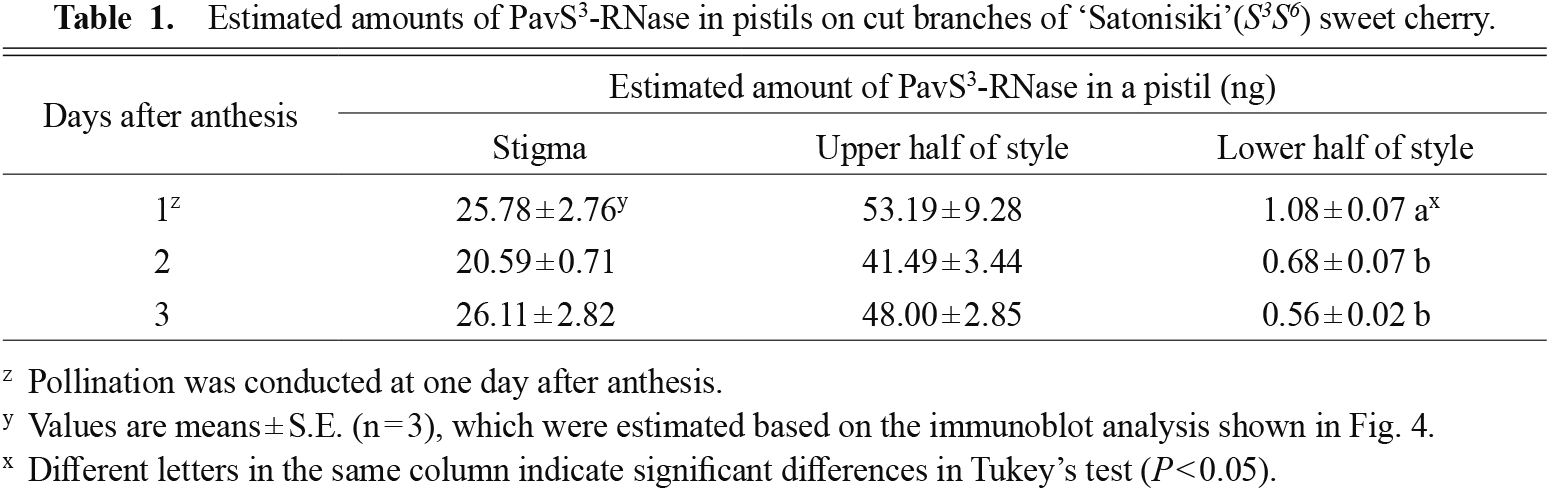
Estimated amounts of PavS3-RNase in pistils on cut branches of ‘Satonisiki’(S3S6) sweet cherry.
The RNase activity of PavS3-RNase was further examined in terms of the stigma and the upper halves of styles with non-reducing SDS-PAGE, followed by RNase activity staining (Fig. 6). Of the two bands showing RNase activity, the upper band was S-RNase and the lower was another RNase. RNase activity of S-RNase was observed in the stigma and the upper halves of styles up to 2 DAA, but disappeared at 3 DAA.
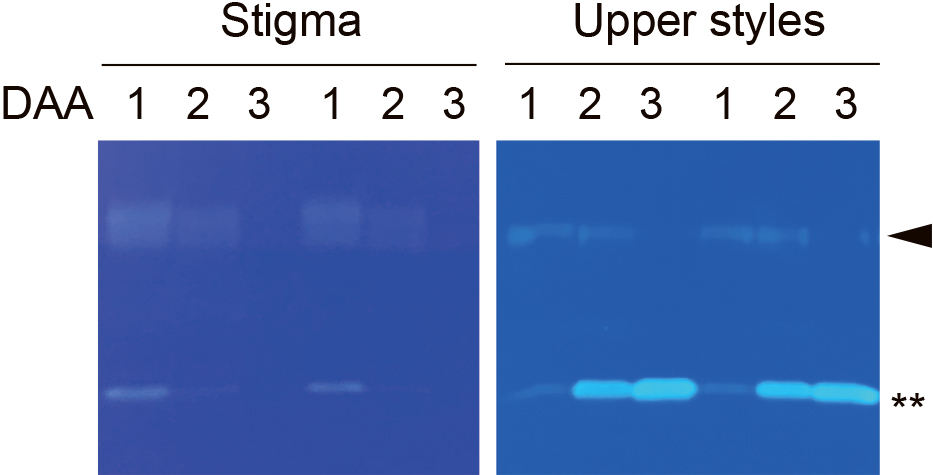
RNase activity staining of stigmas and upper halves of styles of ‘Satonishiki’ (S3S6) at 1–3 days after anthesis (DAA). Crude extracts in each lane were of 5 pistils for the stigma and the upper halves of the styles. An arrowhead indicates S-RNase. A double asterisk indicates another RNase.
We grafted the upper one-third of ‘Satonishiki’ styles pollinated with ‘Rainier’ pollen on different pistils on cut branches at 8 HAP to verify the suppression of incompatible pollen tube growth within styles (Table 2). The pollen tubes of ‘Rainier’ elongated to 7.36 mm 16 h after style-grafting on ‘Satonishiki’ pistils (compatible pistils), but elongated only to 6.57 mm 16 h after style-grafting on ‘Taishonishiki’ pistils (incompatible pistils, P < 0.05). The elongation rates of pollen tubes that were compatible with scion-styles were estimated to be 251 μm·h−1 and 201 μm·h−1 for compatible and incompatible rootstock-pistils, respectively.

‘Rainier’(S1S4) pollen tube growth in the style-grafted pistils 16 h after grafting.
We also grafted the upper one-third of ‘Satonishiki’ styles pollinated with ‘Satonishiki’ pollen on different pistils on cut branches at 16 HAP to examine the reversibility of suppression of incompatible pollen tube growth (Table 3). The pollen tubes of ‘Satonishiki’ elongated to 5.69 mm 16 h after style-grafting on ‘Satonishiki’ pistils (incompatible pistils) and reached 6.79 mm 16 h after style-grafting on ‘Taishonishiki’ pistils (compatible pistils, P < 0.05). The elongation rates of pollen tubes that were incompatible in the scion-style were estimated to be 215 μm·h−1 and 301 μm·h−1 for incompatible and compatible rootstock-pistils, respectively.
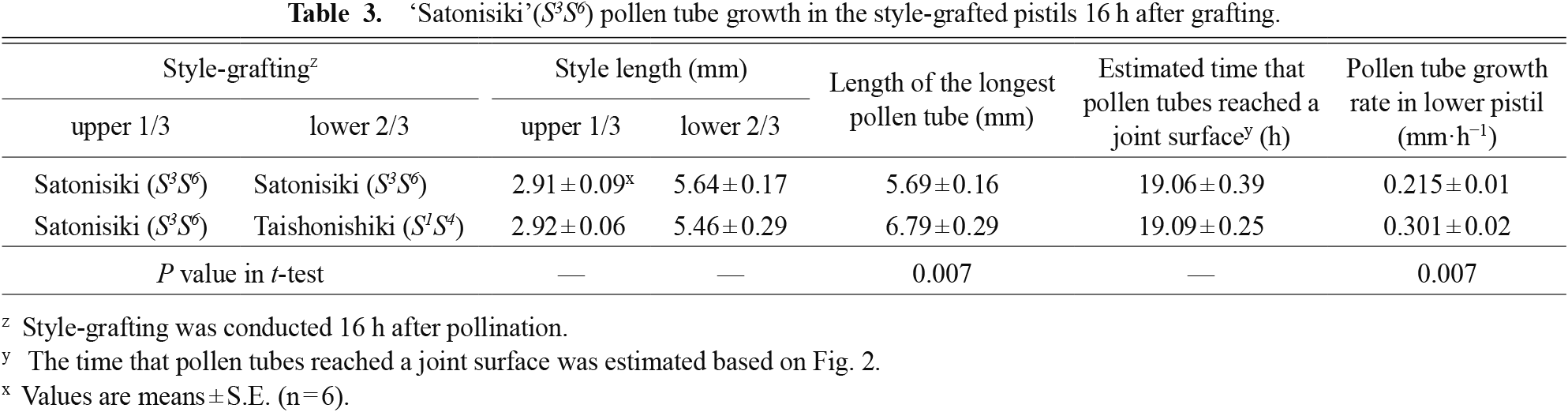
‘Satonisiki’(S3S6) pollen tube growth in the style-grafted pistils 16 h after grafting.
In Solanaceae and Maleae of the Rosaceae family, it has been reported that incompatible pollen germinated and elongated normally until the halfway point the same as compatible pollen, but elongation gradually became delayed and eventually stopped. In this study, we showed that incompatible pollen of P. avium ‘Satonishiki’ already delayed germination or penetration into the stigma, and its pollen tube elongation stopped halfway through styles for a while, and eventually restarted and reached the base of the styles. The pistils on cut branches were almost abscised at 4 DAA when incompatible pollen tubes reached the ovary, indicating that suppression of germination, penetration into stigma, and elongation in the style would be sufficient to inhibit self-fertilization. The effect of genetic background other than S haplotypes on these observations was unclear because reciprocal crossing could not be conducted due to the limitations of our plant materials. Nevertheless, these observations suggest that the manner of self-pollen rejection in Prunus differs from that in Solanaceae and Maleae and that cell death is not involved in self-pollen rejection in Prunus.
Germination or penetration into the stigma of incompatible pollen tubes was delayed by approximately 6 h, contributing greatly to the difference in pollen tube length between compatible and incompatible pollen at the same HAP (Figs. 2 and 3). Estimation of PavS3-RNase amounts in ‘Satonishiki’ styles indicated an abundance of S-RNase in the upper halves and stigma (Table 1). Considering the volume of the tissues, S-RNase concentration was assumed to be the highest in the stigma. Although we previously showed that S-RNase purified from styles of ‘Satonishiki’ suppressed elongation of self-pollen tubes in vitro but not germination, the cocktail of recombinant PavS3-RNase and PavS6-RNase was also confirmed to suppress germination of incompatible pollen in vitro at high concentrations (Matsumoto et al., 2019, 2023). Taken together, the delay in incompatible pollen tubes passing through the stigmas may be caused by the high S-RNase concentration in the stigmas. It is still unclear as to why germination and penetration into the stigma of self-pollen were not observed to be suppressed in Solanaceae and Maleae, in which S-RNase has been confirmed to be localized in the stigma and upper parts of styles (Certal et al., 1999; McClure et al., 1993). One possibility is the difference in the sensitivity of pollen, and another is the difference in the incompatible reaction mechanism. Solanaceae and Maleae have been reported to require pistil factors other than S-RNase to reject self-pollen (modifiers), whereas the existence of a pistil-side modifier has not been identified in Prunus (Hancock et al., 2005; Jiménez-Durán et al., 2013; McClure et al., 1999; Moriya et al., 2009; Torres-Rodríguez et al., 2020). The localization of modifiers, not only that of S-RNase, is related to the position of incompatible pollen suppression.
The elongation rate of incompatible pollen tubes after passage in the stigma was approximately 90% of that of compatible ones, and style-grafting of compatible styles on incompatible pistils decreased the pollen tube elongation rate in the rootstock-pistils, suggesting that self-S-RNase also suppressed pollen tube elongation (Figs. 2 and 3; Table 2). Considering that S-RNase was localized much more abundantly in the upper half of the style, we assumed that enough S-RNase was localized between the region flanked by the border of the top 1/3 to the 2/3 and the border of the top 1/2 and the bottom 1/2 of styles to suppress incompatible pollen tube growth (Table 1). The suppression of pollen tube elongation appeared to be reversible, since style-grafting of incompatible styles on compatible pistils accelerated the pollen tube elongation rate of ‘Satonishiki’ at the rootstock-pistils (Table 3). Interestingly, the elongation rate (301 μm·h−1) was faster than that of ‘Rainier’ pollen tubes elongating in ‘Satonishiki’ pistils. A kind of acclimation to S-RNase may have occurred in incompatible pollen, allowing incompatible pollen tubes to detoxify “non-self” S-RNase effectively.
Incompatible pollen tubes stagnated at approximately 70% of the total length of styles from 32 HAP to 48 HAP, at which point there was only a little S-RNase (Table 1). Interestingly, the RNase activity of S-RNase in the stigma and upper halves of styles decreased at 3 DAA, which corresponds to the 48 HAP when incompatible pollen tubes restarted elongation (Fig. 6). Therefore, this stagnation may be a result of the accumulation of S-RNase taken up into pollen tubes, and be released by conformational collapse of S-RNase, represented by RNase activity at 3 DAA.
In Maleae, S-RNase was shown to bind and depolymerize F-actin in incompatible pollen tubes at a reversible stage of inhibition, which suggested irreversible programmed cell death would eventually be triggered (Chen et al., 2018). Prunus S-RNase has also been shown to bind to F-actin in vitro (Matsumoto and Tao, 2012). Further studies are required to examine whether disorganization of the F-actin cytoskeleton is related to a delay in germination, penetration into stigma, and elongation of pollen tubes, as well as to why the Prunus SI reaction lacks irreversible inhibition; this information will clarify the diversity and commonality of S-RNase-based self-incompatibility mechanisms.
Extensive research has been conducted to control Prunus SI artificially and these studies indicated that artificial control of Prunus SI is very challenging. Although promotion of self-pollen tube growth was observed with reduced innate RNase activity by hot water treatment of P. × yedoensis styles, none of the fruit was set (Tsuruta et al., 2020). Ono et al. (2022) recently succeeded in overcoming SI by using an antisense oligo against S haplotype-specific F-box (SFB) and M-locus-encoded glutathione S-transferase-like (MGST). Although a few selfed progenies were obtained in P. salicina and P. mume, the efficiency was very low as only approximately 0.3% of the pollinated flowers set fruit. Our findings in this study that the Prunus incompatible reaction is reversible and the use of style-grafting give us important clues to develop artificial controls for Prunus SI.