2017 年 57 巻 5 号 p. 883-887
2017 年 57 巻 5 号 p. 883-887
Diffusion bonding process in low temperature is desirable for the manufacturing method of metal MEMS (Micro-Electronic-Mechanical Systems) such as metal micro-pump that the high proof stress is required. Severe plastic deformed metals having high grain boundary mobility are expected to bond in low temperature. Then, we tried to perform recrystallization and solid phase diffusion bonding at the same time. In this paper, we confirmed that the reduction of diffusion bonding temperature in severe plastic deformed SUS304 and SUS316L as compared to solution heat treated one. Especially the bonding temperature was decreased prominently in SUS304 having strain-induced martensite.
To produce a fine, precise structure required in micro-electronic-mechanical systems (MEMS), various methods have been proposed that depend on the type of material used. A recently developed production method of metal MEMS is diffusion bonding after laminating the metallic foil that had been shaped to planar form by etching.1,2) In the case of austenitic stainless steel, the high bonding temperature of approximately 1200 K causes annealing of materials, resulting in a decrease in proof stress. Although this decrease should not be a serious problem for a component such as a heat exchanger, for components that include driving parts, such as a metal micro-pump, it might cause defects in the valve operation with low elasticity.
Such high bonding temperature requires long heating and cooling times. Moreover, the components of the bonding apparatus must have high thermal resistance. These difficulties can be resolved by the development of a diffusion bonding method at lower temperature.
For this study, we demonstrated the bonding of a severely plastic-deformed austenitic stainless steel at lower bonding temperature with solution treatment. Results revealed an ultra-fine grain size less than 1 μm in these bonded samples, which is expected that indicating high proof stress in the bonding components.
The austenitic stainless steel samples were SUS304 and SUS316L bars (40-mm square) of commercial purity whose chemical composition is listed in Table 1. Bars of each type were solution-treated for 2 min at 1353 K, and then cut into 20-mm long billets for thermomechanical treatment. The treatment conditions for each sample are shown in Table 2.
| C | Si | Mn | P | S | Mo | .Co | Ni | Cr | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|
| SUS304 | 0.05 | 0.48 | 0.97 | 0.035 | 0.004 | – | 0.19 | 8.02 | 18.09 | bal. |
| SUS316L | 0.021 | 0.50 | 1.49 | 0.034 | 0.002 | 2.01 | – | 12.10 | 16.91 | bal. |
| Material | Name | Temperature of 90% rolling after warm upsetting | Heat treatment | Remark |
|---|---|---|---|---|
| SUS304 | WC | RT. | – | α’, high strain |
| SUS304/SUS316L | W99 | 573 K | – | γ, high strain |
| SUS304 | WCA | RT | 973 K×1800 s | γ, fine grain size |
| SUS304/SUS316L | SOL | 573 K | 1373 K×600 s | γ, coarse grain size |
The thermomechanical treatment consisted of two steps; upsetting and rolling. In the first step, the billets were subjected to two-directional upsetting at 573 K, in which the strain added was the same as the true strain with 90% single-directional upsetting. At the end of this step, the thickness of each billet was 10 mm.
In the second step, all of the SUS316L billets were warm-rolled at 573 K in to a 1-mm-thick sheet (hereafter called W99 materials), and half of the SUS304 billets were warm-rolled at 573 K (W99 materials) and the other half were cold-rolled at room temperature (WC materials). All of these rolled samples had a final thickness of 1 mm. In the cold-rolling step, the reduction in sheet thickness after one pass was controlled to less than 5% to prevent an increase in temperature by processing heat that inhibits martensitic transformation. The austenitic phase was transformed to martensite in the cold-rolled SUS304 sheet. It was expected that the highly worked martensite would transform into austenitic phase by annealing and that ultra-fine grain size of the austenitic phase would be obtained.3,4) The WC materials were annealed for 1800 s at 973 K. Structures of austenitic phase with fine grain size (WCA materials) were obtained. The other samples of SUS304 and SUS316L (W99 materials) had severely plastic-deformed austenitic phase. For comparison, 1-mm-thick solution-treated sheets of SUS304 and SUS316L (SOL materials) were also prepared.
Figure 1 shows cross-sections of backscattered electron (BSE) images of WCA, WC, and SOL materials of SUS304 obtained using a scanning electron microscope (SEM). The WCA material (Fig. 1(a)) consisted of equiaxial crystals with microstructures in which the grain size was less than 0.5 μm. This material showed superplastisity at 973 K,5) and 0.2% of the material (by volume) was the remaining martensite as measured by a ferrite scope (Fischer Co., Ltd.). The WC material (Fig. 1(b)) had martensite phase with high strain, and the grain boundary was not visible in the image and only the layer structure produced by rolling and by martensitic transformation was observed. The SOL material (Fig. 1(c)) had coarse austenite grains (> 100 μm).

SEM BSE image of SUS304 sheet for diffusion bonding.
For the cross-tensile test, treated specimens were then machined to the shape shown in Fig. 2. Each specimen was 50-mm long, 10-mm wide, and had four 4.2-mm-diameter drilled holes for the bolts used to hold the specimen on the jig of the cross-tensile test. The specimens were 1/5 scale of the cross-tensile test specimen specified in JIS Z3137, but the grip sections were enhanced. One side of each specimen was ground and polished to a mirror-like finish using emery paper. The maximum arithmetic average roughness was 20 nm based on measurements at 10 points on each specimen.

Dimensions of the test piece for bonding test.
For microstructure analysis, treated specimens 10-mm wide and 15-mm long were prepared, and were also ground and polished to a mirror-like finish.
2.2 Bonding ExperimentsPairs of the SOL and W99 (i.e., SOL/SOL and W99/W99) were used in the bonding experiments of SUS316L, and pairs of SOL, W99, WC, and WCA (i.e., SOL/SOL, W99/W99, WC/WC, and WCA/WCA) were used in the bonding experiments of SUS304. In addition, pairs of different pre-treated materials were used in bonding experiments of SUS304, namely, WC/WCA and WC/SOL.
The jig for the bonding experiments (Fig. 3) was composed of a base plate (10-mm thick and 60-mm diameter), a punch guide (10-mm thick and 60-mm diameter) with a 5-mm-diameter drilled hole, and a punch (5-mm diameter). The lower end of the punch that was in contact with the specimen was machined as flat, whereas the upper end of the punch that was in contact with the upper push rod was machined as spherical to correct the gradient passively. All parts of the bonding jig were Inconel-HX alloy. The bonding temperature was measured using a thermocouple spot-welded on the bottom of the base plate that was in contact with the outer surface of the lower push rod. The jig was enclosed in a chamber for controlling the surrounding atmosphere.

Outline of the jig for diffusion bonding.
A pair of the polished specimens was set into the jig. The specimens were crossed and their polished surfaces were in contact with each other. The atmosphere in the chamber was evacuated to less than 3×10−3 Pa, and a load of 980 N (50 MPa for 5-mm diameter) was set. The stress selected was lower than the flow stress at the superplastic deformation of the specimen material at the condition of 1×10−4/s at 973 K.6) The specimen in the jig was then heated to a set bonding temperature using high-frequency induction heating. During the bonding, the added load and bonding temperature were maintained for 1800 s. The bonded specimen was then cooled to 573 K under the vacuum condition, and then air was introduced into the chamber to cool the specimen to room temperature. The cross-tensile tests were then executed on the specimen.
The specimens for microstructure analysis were bonded using 20-mm-diameter flat dies. A 10 mm×10 mm square area of each specimen was bonded. The protocol of the bonding temperature and stress for the bonding was the same as that for the cross-tensile test.
The cross-section of each bonded specimen was polished by electro-polishing, and the microstructure was observed by SEM and analyzed by electron backscatter diffraction (EBSD).
Figure 4 shows the relationship between the fracture load in the cross-tensile test and the bonding temperature for the WC/WC, W99/W99, and SOL/SOL specimens of SUS304. The WC and W99 materials can apparently bond at lower temperature compared with the SOL material. The reduction in the bonding temperature is greater for WC than for W99, indicating that the addition of strain as well as the presence of martensite reduces the bonding temperature. Figure 5 shows the measured fracture load vs. bonding temperature for the WC/WCA and WC/SOL of SUS304, along with that for WC/WC, SOL/SOL, and WCA/WCA. Compared with SOL/SOL, the other materials had lower bonding temperature. There was no significant difference in the results between WC/WCA and WCA/WCA. In contrast, at comparatively low bonding temperature ,WC/SOL showed a higher fracture load than did WC/WC.

Bonding strength on bonding test at SUS304 induced martensite and strain.
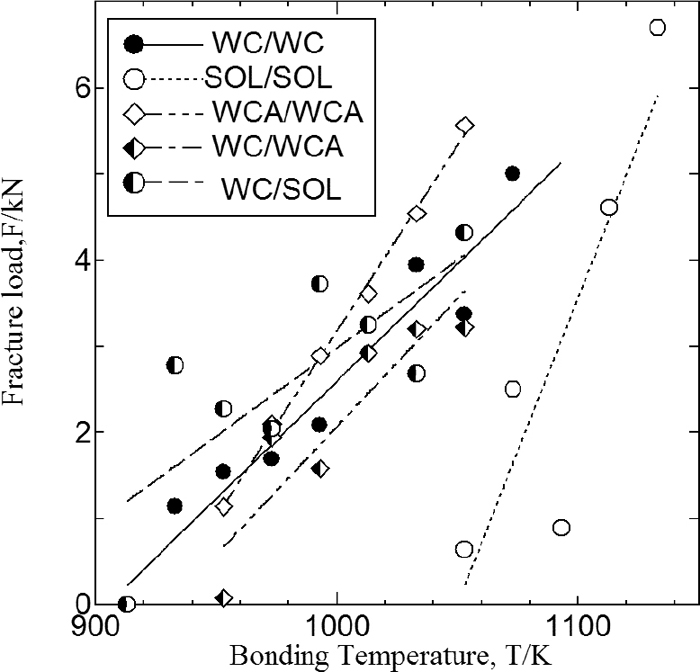
Bonding strength on bonding temperature at SUS304 as various pretreated.
Figure 6 shows the cross-sectional inverse pole figure (IPF) mapped by EBSD of WC/WC bonded at 973 K. The grain boundaries continue almost in a straight line, but some grains have grown over this line, and the line twists and turns slightly, and then the line shifts from the straight line. The grains that are generated on the bonding plane are also shown.
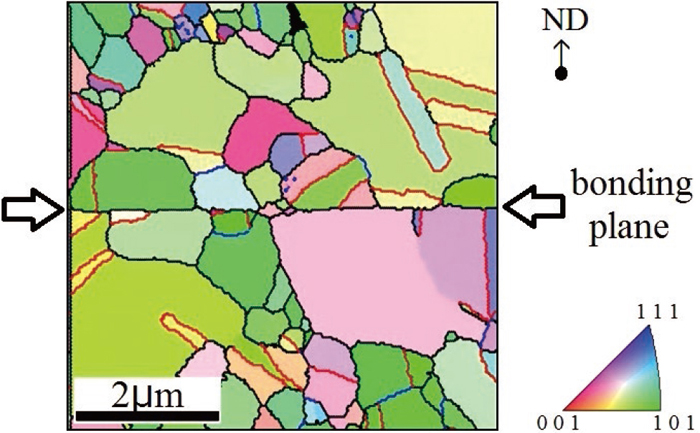
Inverse pole Figure map near the bonding interface of SUS304 (WC treated).
Figure 7 shows SEM images of the fractured surface after the cross-tensile test for WC/WC and WCA/WCA (bonded at 1033 K). For WC/WC, the fracture mode is a base material fracture, and a 5-mm-diameter “dent” can be seen with the naked eye on one of the surfaces. The fracture surface shows figuration, i.e., the layer structure appears “torn” at low magnification, but appears dimpled at high magnification. In contrast, for WCA/WCA, the fractured surface is similar to the surface before bonding, but has an oval-shaped cup (approximately 10 μm as the longest diameter) appearing on the flat surface at low magnification. At high magnification, a row of small dimples (a few nm in diameter) appear along with a mesh structure schematically illustrated below the image. The mesh size is similar to the grain size of the WCA materials (Fig. 1). These results for both the WC/WC and WCA/WCA suggest that the parts of the grain boundary exposed on the bonding surface are selectively bonded, and that the inner parts of the crystal grains that are exposed on the bonding surface did not bond. There are two explanations for this observed structure on the fracture surface. One is tilting of the bonding surface of each grain due to sliding of the grain boundary. The part of the bonding surface for which grains are exposed, is tilted due to this sliding of the grain boundary, and thus the heaved edge of the grain becomes bonded. The other explanation is the higher activity of the grain boundary diffusion than that of intraparticle diffusion. It is thought that this higher activity encourages bonding at the mesh part of the grain boundary that is exposed.

Fracture surface at peeling test of diffusion bonded SUS304.
Figure 8 shows the results of the cross-tensile test for W99/W99 and SOL/SOL at SUS316. Similar to the case of SUS304 (Fig. 4), the bonding temperature is lower for severely plastic-deformed material (W99).
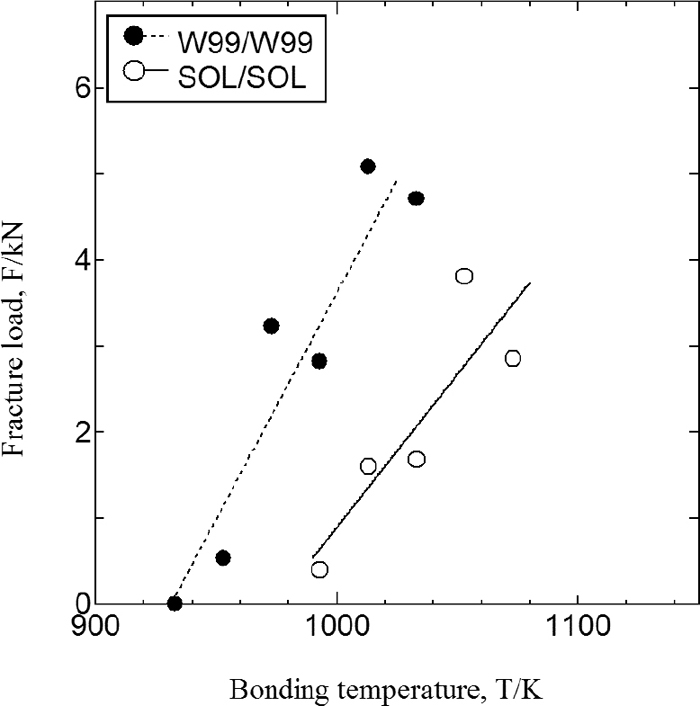
Bonding strength on bonding temperature at SUS316L induced strain.
Harumoto et al.7) reported the reduction of the bonding temperature by pre-working the austenitic stainless steel, but did not report a mechanism responsible for the reduction in bonding temperature. Moreover, for titanium alloys, Han et al.8) regarded the mechanism as an increase in the grain boundary diffusion, which leads to fine grains due to localized severe plastic deformation and recrystallization. Almost all of our results in our current study can be potentially explained by the study of Han et al. However, one of our results that needs another explanation is that the fracture load for WC/SOL, in which a material has very coarse grain size, is higher than WC/WCA or WC/WC at comparatively low bonding temperature.
Figures 9 and 10 show the Vickers hardness of W99 and WC of SUS304 at the annealing temperature of 1800 s, and Fig. 11 shows that of W99 of SUS316L. The hardness is an average of data measured at five locations on each specimen. Figure 10 also shows the volume fraction of the remaining martensite. The temperatures at which recrystallization started and finished are evident from these figures. For SUS304, the start and finish temperatures were approximately 800 K and 1100 K, respectively, independent of the presence or absence of martensite. For SUS316L, the temperatures were approximately 900 K and 1200 K, respectively. For both SUS304 and SUS316L, the temperature at which bonding started was between their respective recrystallization start and finish temperatures, suggesting that the bonding behavior in these experiments is linked to recrystallization.
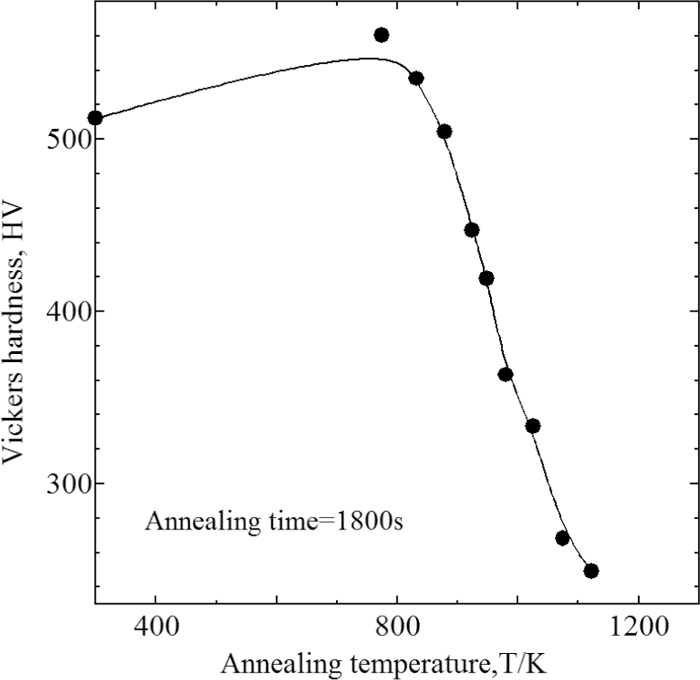
Change of Vickers hardness on annealing temperature at SUS304 (W99 treated).
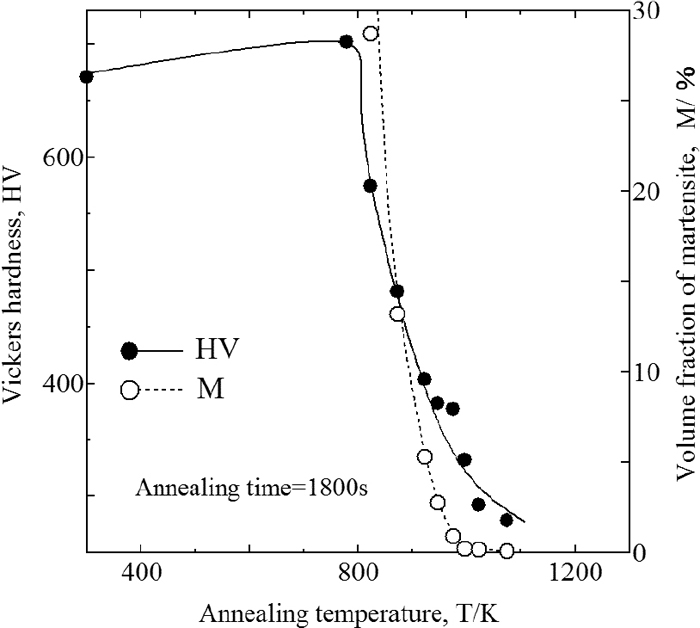
Change of Vickers hardness and remained martensite on annealing temperature at SUS304 (WC treated).
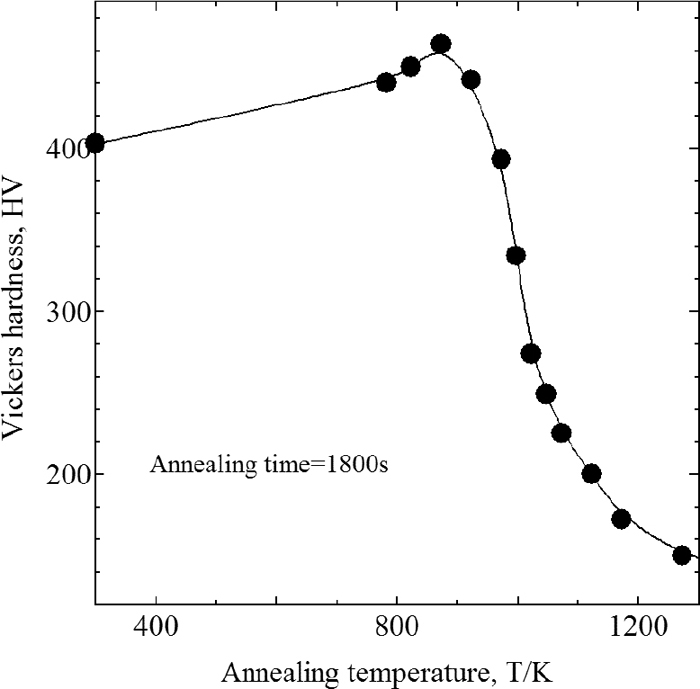
Change of Vickers hardness on annealing temperature at SUS316L (W99 treated).
Diffusion bonding is believed to require the breaking of the inhibitory layer of a non-metallic atom, such as oxygen, on the bonding plane by atomic diffusion and to require contact between each bonding plane. At a high enough temperature, atomic diffusion of each grain boundary and intraparticle occurs and oxygen in the inhibitory layer is absorbed by the base material, thus enabling diffusion bonding to occur. If the temperature decreases, the activity of atomic diffusion decreases, and consequently the inhibitory layer cannot be broken and diffusion bonding does not occur. However, the energy of the activation at a grain boundary is generally lower than the intraparticle energy. Therefore, atomic diffusion is considered to be active at lower temperature, and oxygen is removed near the grain boundary that appears on the bonding plane, and thus diffusion bonding occurs at that location. The mesh structure of the dimples on the fracture surface in the cross-tensile test for WCA/WCA of SUS304 is therefore apparently caused by the diffusion bonding only near the grain boundary.
Furthermore, intraparticle atomic diffusion is believed to be active in restructuring the atomic arrangement at a condition where recrystallization occurs. In our bonding experiments for WC/WC of SUS304, the entire surface was bonded independently of the grain boundary on the fracture surface. This suggests that oxygen in the inhibitory layer was absorbed into the base material, and activated intraparticle diffusion occurred by the addition of strain even at relatively low temperatures for which intraparticle diffusion does not occur without strain. Furthermore, it is expected that the inhibitory layer was broken either by growth of recrystallized grains over the bonding plane or by the generation of recrystallized grain at the bonding plane as shown in Fig. 6.
The larger decrease in the bonding temperature for WC/SOL than for either WC/WC or WC/SOL {CP53}is possibly due to a mechanism such as Ostwald ripening, and to coarse grains growing far over the bonding plane. Our experiments are not sufficient to determine this mechanism. On the other hand, WC/WCA, respectively a pair of severely plastic-deformed martensite and austenite phase with fine grain size, showed no significant difference in than that for WC/WC, because the driving force for growth of the austenite grain in WC/WCA is weaker than that in WC/SOL.
In summary, diffusion bonding is apparently accelerated by introduction of martensite or hard plastic strain. A decrease in bonding temperature from the addition of plastic strain is primarily caused by diffusion bonding in the entire bulk. A minor contribution to this decrease is acceleration of grain boundary diffusion by grain refinement.
In this study, we demonstrated the diffusion bonding with inducing plastic strain and martensite to austenitic stainless steel SUS304 and inducing of plastic strain to SUS316L. The strain-introduced material showed lower bonding temperature, accompanied with recrystallization of severely plastic-deformed material. When martensite was added, the bonding temperature became even lower. These results suggest that nucleation of the austenite phase recrystallization is aided by reverse diffusion type transformation of martensite to austenite phase. However, when the material had austenitic phase with ultra-fine grain, only the area close to the grain boundary exposed on the bonding surface was bonded.