2013 Volume 60 Issue 2 Pages 117-121
2013 Volume 60 Issue 2 Pages 117-121
Deproteinized wheat starch (Act-WS) was prepared by twice digesting with actinase large granular wheat starch classified from wheat starch that had been extracted with 70% ethanol. A small amount of preoteinaceous component still remained in Act-WS. The effect of lysine (Lys), monosodium glutamate (GluNa), glycine (Gly) and alanine (Ala) on the thermal behavior of Act-WS, and the dispersion state of the swollen starch granules in the Act-WS paste was investigated. Consequently, Gly and Ala had little effect on the gelatinization temperature and viscosity, whereas GluNa and Lys markedly elevated these parameters. This increased viscosity with Lys contrasted the results for potato starch. Such charged amino acids as GluNa and Lys reduced the swelling and solubility, while Lys elevated the turbidity, median diameter and ratio of aggregates of the swollen granules. The increased viscosity was thus caused by forming aggregates with Lys, presumably through interacting with a starch-granule-associated protein.
Starch is widely used to build or modify the physical properties of many processed foods. Since starch naturally occurs as granules that are insoluble in cold water, these functional properties can only be revealed in the gelatinized state through the absorption of a large quantity of water at a characteristic temperature by heating in water. However, the swollen starch granules easily break down under physical stress, resulting in a substantial viscosity change and the development of a sticky and pasty texture that is disliked in starchy foods. This starch paste gradually becomes cloudy with cooling, and the viscosity increases due to partial recrystallization of the starch chains, resulting in a deterioration of the quality of starchy foods. It is thus very important to control the gelatinization behavior so as to maintain the condition of the swollen starch granules and to reduce the degradation of the physical properties during subsequent cooling. A number of studies have been carried out to investigate the effects of many coexisting substances on the gelatinization behavior. We have specifically paid attention to amino acids, and clarified that such charged amino acids as lysine (Lys) and monosodium glutamate (GluNa) strongly elevated the gelatinization temperature, and markedly reduced the viscosity and swelling of potato starch, in contrast to such zero net charge amino acids such as glycine (Gly) and alanine (Ala),1) as had also been reported by Ling and King.2) These effects had been demonstrated to depend strongly on the absolute value of the amount of their net charge,3) and to be due to the binding strength of the amino acid to the starch chains.4) It has been also reported that the charged amino acids could inhibit collapse of the swollen starch granules, even when retorted.5)
These findings were obtained by using potato starch as the experimental starch sample. Potato starch is well known to have substantially little proteinaceous content, being amphoteric electrolyte like amino acids, when compared with other starches. For example, wheat starch, the main component of many wheat products such as pasta, bread and fried coatings, has a relatively large proteinaceous content including a starch-granule-associated protein called friabilin. 6) If added amino acids could create interaction with the starch-granule-associated protein, some connectivity among the swollen starch granules could appear when heated. Consequently, wheat starch may not always reveal the same thermal behavior with amino acids as potato starch does.
The objectives of this present study are to clarify the characteristic effects of charged amino acids on the thermal behavior of wheat starch in terms of the gelatinization behavior, the pasting behavior, swelling index and solubility, and dispersion state of the swollen starch granules in the gelatinized suspension.
Materials. Soft wheat flour (Violet, 14% moisture; 7.1% protein and 0.33% ash) was obtained from Nisshin Flour Milling Inc. (Tokyo, Japan). Glycine (Gly), Alanine (Ala), monosodium glutamate (GluNa) and lysine (Lys) were obtained from Ajinomoto Co. (Tokyo, Japan) and Actinase E, non-specific proteases, was purchased from Kaken Pharmaceutical Co. (Tokyo, Japan). All other reagents were commercially available.
Preparation of the classified wheat starch. Soft wheat flour (50 g) was well mixed by hand with 25 mL of distilled water at room temperature for 3 min, and the dough ball was wrapped in wet gauze. After resting at room temperature for 30 min, and then the resulting dough ball was thoroughly washed out by hand in distilled water to separate the wheat starch. The dispersion was then centrifuged at 3,800×G for 5 min. The resulting precipitate was re-dispersed in distilled water, and again centrifuged. This treatment was conducted six times to obtain the crude wheat starch. This crude wheat starch was extracted with 100 mL of 70% ethanol at room temperature for 5 h to eliminate the alcoholic soluble protein, and then recovered by filtering with glass fiber filter (Advantec Co., Ltd.,, Tokyo, Japan) under reduced pressure. The recovered starch was dispersed in distilled water and allowed to form a precipitate. This treatment was conducted six times to eliminate ethanol, and the precipitate was air-dried to obtain wheat starch (WS).
WS was classified as follows. WS was dispersed in a double volume of distilled water and then filtered through a double layer of gauze. The resulting suspension was diluted with the same volume of distilled water, and then placed for about 30 min until about half the solid had precipitated. The upper dispersion was separated by decantation. The lower precipitated fraction obtained was re-dispersed in double the volume of distilled water, and stood for about 30 min to obtain the precipitate. This classification was repeated until the resulting precipitate was composed of only large granules without any small granules, this being confirmed by microscopic observation. The final precipitate was collected by filtering though a glass fiber filter (Advantec Co., Ltd.) under reduced pressure, and air-dried as a large granular wheat starch (LG-WS). The upper dispersion was filtered through the glass fiber filter under reduced pressure, and then the resulting residue was re-dispersed and stood. This process was repeated in the same manner until the dispersion was composed of only small granules without any large granules. This final dispersion was filtered through the glass fiber filter under reduced pressure, and the resulting starch was air-dried as small granular wheat starch (SG-WS). Each starch sample was passed through 100-mesh screen before being used.
Preparation of deproteinized WS. Removal of protein from LG-WS was carried out by digesting with actinase according to the previous method7) with some modifications. Actinase E (1 g, Kaken Pharmaceutical Co.) was dissolved in 30 mL of 1/15 M phosphate buffer (pH 7.0), and filtered through the glass fiber filter (Advantec Co., Ltd.). The filtrate was diluted to 160 mL with the same buffer to obtain an Actinase solution. After 20 g of LG-WS had been added to this actinase solution, the reaction mixture was incubated at 25°C for 24 h while shaking, and then 30 mL of a fresh actinase solution was added to continue actinase-digestion for 24 h more. The reaction product was recovered by filtration and then thoroughly washed with distilled water by repeating the water-dispersion and sedimentation process six times. After air-drying, Actinase-digested LG-WS (Act-WS) was obtained as the deproteinized WS preparation. The starch sample was passed through a 100-mesh screen before being used.
Differential scanning calorimetry (DSC). After adding 10 μL of 1.12 M amino acid, which had been adjusted to pH 7.0 with 0.1 M HCl or 0.1 M NaOH, to 5 mg of the starch sample in an airtight anodized aluminum capsule, DSC was conducted to determine the gelatinization temperature and enthalpy by using an SSC-5020 DSC-6100 instrument (SII NanoTechnologies Inc., Chiba, Japan) as previously described.8) Distilled water (15 μL) was used as a reference. Each sample was heated from 5 to 100°C at 2 K/min. Triplicate measurements were taken.
Viscosity measurement. After adding 25 mL of 1.12 M amino acid, which had been adjusted to pH 7.0 with 0.1 M HCl or 0.1 M NaOH, to 2.0 g of the starch sample in an aluminum container, an RVA Super3 Rapid ViscoanalyzerTM (Newport Scientific Pty Ltd., Warriewood, Australia) was used to measure the pasting properties. Triplicate RVA measurements for potato starch had shown a high degree of reproducibility, the coefficient of variation of the peak viscosity being evaluated as only 0.19%,9) so each measurement was taken only once in this study.
Measurement of the swelling index and solubility. The swelling index of the starch sample was measured as previously described.1) In brief, the starch sample (0.1 g) in a polypropylene centrifuge tube (15 i.d.×60 mm) with a lid was suspended in 5 mL of 0.1 M Gly and Lys, which had been adjusted to pH 7.0 with 0.1 M NaOH or 0.1 M HCl, and then heated in an aluminum block bath (Scinics Corporation, Tokyo, Japan) at 93°C for 30 min while stirring at 500 rpm. After quickly cooling to room temperature, the sample was centrifuged at 31,000×G for 30 min at 20°C. The resulting supernatant was completely removed, and the dissolved saccharide was determined by the phenol-sulfuric acid method10) to evaluate its solubility. The precipitate was weighed (Ww) and then dried at 110°C for 16 h to obtain a constant weight (Wd). The swelling index was calculated by the following equation:
Swelling index = Ww/Wd
Triplicate measurements were taken.
Measurement of the turbidity of the gelatinized starch suspension. Act-WS (5 mg) was heated in 5 mL of 0.1 M Lys (pH 7.0) at 50, 60, 70, 90 and 100°C for 20 min while stirring at 160 rpm. The turbidity of the gelatinized Act-WS suspension was evaluated by measuring the absorbance at 800 nm with a UV-160A spectrophotometer (Shimadzu Corp., Kyoto, Japan) to evaluate the dispersion state of the swollen starch granules according to the previous method5) with some modifications.
Microscopic observation. Wheat starch (3 mg) was heated for 20 min in 3 mL of 0.1 M Lys (pH 7.0) at 60, 70, 80 and 90°C while stirring at 160 rpm. A PM-10AD polarizing microscope (Olympus Corp., Tokyo, Japan) was used to evaluate the aggregated swollen starch granules at a direct magnification of 100 times after staining with iodine. Microscopic evaluation was done by using ten visible fields that had been randomly selected.
Measurement of the granular size distribution. The granular size distribution of the native and gelatinized starch samples was evaluated by a Sald-2100 laser diffraction particle size analyzer (Shimadzu Corp.) according to the previous method.11) The native starch sample was dispersed in distilled water. After sonicating for 30 s, the dispersion was diluted with distilled water until the absorbance at 500 nm reached within 0.1‒0.2, and the particle size was evaluated while agitating. The gelatinized sample was prepared by heating a 0.1% suspension of 0.1 M Gly or Lys (pH 7.0) at 70, 90 and 100°C for 20 min while stirring at 160 rpm. Five measurements were taken.
Analytical methods. The total sugar content was determined by the phenol-sulfuric acid method10) as glucose. The nitrogen content of each wheat starch preparation was measured by a Sumigraph NC 22A analyzer (Sumika Chemical Analysis Service Ltd., Tokyo, Japan), using acetanilide as the standard as previously described,3) and the protein content was evaluated by using a conversion factor of 5.7.
Statistical analysis. The multiple comparison method of Bonferroni/Dunn was used to compare the mean values at the 5% level of significance.
Features of the wheat starch preparations. Wheat starch (WS) was separated from soft wheat flour dough by washing out with distilled water. Crude WS obtained had a small amount of protein (Table 1). WS prepared from crude WS by extracting the alcoholic soluble protein with 70% ethanol showed a significantly lower protein content. WS was classified into the large granular WS (LG-WS) and small granular WS (SG-WS) preparations by the rate of sedimentation. Each granular size showed proper classification (Table 1), LG-WS having a significantly lower protein content than that of SG-WS. LG-WS was thus further deproteinized by digesting twice with actinase to obtain actinase-digested LG-WS (Act-WS). However, Act-WS had still a little amount of protein (0.12%). This indicates the difficulty in complete deproteination in such a mild manner without damaging the starch granules. Since such protein as friabilin occurs as a starch-granule-associated protein, probably through hydrophobic and ionic interactions,6) digestion with protease may reach a limit, probably due to steric hindrance by the great bulk of the starch granules. Even Act-WS was thus different from potato starch with only little protein content (< 0.1%).

Features of the wheat starch preparations.
Crude WS, wheat starch separated from wheat dough; WS, wheat starch purified by extracting crude WS with 70% ethanol; LG-WS, large granular wheat starch classified from WS; SG-WS, small granular wheat starch classified from WS; Act-WS, twice actinase-digested LG-WS. Each value is the mean ± SD (n= 3). Different letters show significant difference (p < 0.05). *Based on dry basis. **Duplicate measurements.
The gelatinization properties of the wheat starch preparations were confirmed by DSC. LG-WS showed significantly lower gelatinization temperatures (Tp and Tc) than those of SG-WS (Fig. 1) , corresponding to the previous results,12) whereas Act-WS showed significant increase in gelatinization temperatures and a narrow transition (Tc‒To) compared to those of LG-WS. This suggests that Act-WS had a higher homogeneously ordered structure. There were no significant differences in enthalpy among WS, LG-WS and SG-WS, while Act-WS showed significantly lower enthalpy than the others.

Thermal characteristics of the classified wheat starch preparations evaluated by DSC.
WS, wheat starch purified by extracting crude WS with 70% ethanol; LG-WS, large granular wheat starch classified from WS; SG-WS, small granular wheat starch classified from WS; Act-WS, actinase-digested LG-WS. Different letters show significant difference (p < 0.05).
Characteristic thermal behavior with charged amino acids. The gelatinization behavior of LG-WS and Act-WS in 1.12 M Gly, Ala, GluNa and Lys was evaluated by DSC. The effect of an amino acid on the gelatinization behavior of potato starch has been reported to increase with their concentration,1) indicating the advantage of a higher concentration for evaluating the characteristic gelatinization behavior with amino acids. However, since the solubility of Ala was lowest among the four amino acids, the amino acid concentration in the present study was prescribed to be 1.12 M, close to the solubility of Ala. Gly and Ala had a significant but relatively weak effect on increasing the gelatinization temperature of LG-WS, whereas GluNa and Lys had a strong influence (Fig. 2). This result indicates that the charged amino acid was superior for increasing the thermal stability of LG-WS to that of a zero net charge amino acid, probably due to stabilization of the starch structure by electrostatic interaction as has previously been described in verifying the contribution of the controlling effects of amino acids on the gelatinization behavior of potato starch.4) Gly and Lys were used in the subsequent experiments, because the effects of Ala and GluNa were similar to those of Gly and Lys, respectively. These amino acids also worked in a similar manner on Act-WS (Fig. 2) .

Effect of amino acids (1.12 M) on the gelatinization temperature of LG-WS and Act-WS evaluated by DSC.
 , control without an amino acid;
, control without an amino acid;  , Gly;
, Gly;  , Ala;
, Ala;  , GluNa;
, GluNa;  , Lys. Different letters show significant difference (p < 0.05). Any omitted error bars were too close together to show.
, Lys. Different letters show significant difference (p < 0.05). Any omitted error bars were too close together to show.
The pasting behavior of Act-WS with 1.12 M Gly, Ala, GluNa and Lys was investigated by RVA. Gly and Ala had substantially no or little effect on the peak viscosity (PV) and breakdown (BD) of LG-WS, whereas GluNa and Lys substantially increased PV and reduced BD (Fig. 3). These results also indicate that the amino acids with zero net charge had little effect on the wheat starch, while the charged amino acids exhibited a strong effect. However, the effect of the charged amino acids on PV of LG-WS and Act-WS was clearly different from that of potato starch, because the PV value for potato starch was markedly reduced by the charged amino acid due to the decreased swelling.1) In subsequent studies, Act-WS with the lowest proteinaceous content was used to avoid the effects of starch-granule-associated protein as much as possible.

Effect of amino acids (1.12 M) on the pasting behavior of LG-WS and Act-WS evaluated by RVA.
 , control without an amino acid;
, control without an amino acid;  , Gly;
, Gly;  , Ala;
, Ala;  , GluNa;
, GluNa;  , Lys. PV, peak viscosity; BD, breakdown.
, Lys. PV, peak viscosity; BD, breakdown.
The swelling and solubility of Act-WS heated at 93°C for 30 min was measured, because the increased PV value with Lys suggested an increase in the swelling. In this experiment, an amino acid concentration of 0.1 M was selected to avoid any measurement error due to the amount of amino acid retained by the starch granules. The swelling index remained unchanged or somewhat reduced with Gly or Ala, respectively, whereas GluNa and Lys resulted in a large decrease (Fig. 4). The solubility showed a small decrease with Gly and Ala, and a large decrease with GluNa and Lys (Fig. 4). Swelling and solubility at 70°C also showed similar tendency (swelling index, 6.8 ± 0.16 for control, 6.5 ± 0.03 for Gly and 6.0 ± 0.10 for Lys; solubility, 9.6 ± 0.69 for control, 6.8 ± 0.66 for Gly and 5.0 ± 0.58 for Lys) to those at 93°C. The decreased swelling index and solubility values imply a decrease in the PV value, not an increase. The reason for the increase in the PV value thus requires verifying by more work.
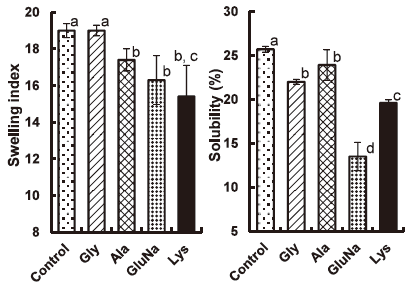
Effect of amino acids (1.12 M) on the swelling index and solubility of Act-WS heated at 93°C for 30 min while stirring at 500 rpm.
 , control without an amino acid;
, control without an amino acid;  , Gly;
, Gly;  , Ala;
, Ala;  , GluNa;
, GluNa;  , Lys. Different letters show significant difference (p < 0.05).
, Lys. Different letters show significant difference (p < 0.05).
Characteristic dispersion state of the swollen starch granules with charged amino acid. The Act-WS suspension containing 0.1 M Lys and control suspension without Lys were heated at 50‒100°C for 20 min. Since their absorption spectra from 400 to 800 nm indicated no characteristic absorption (data not shown), and the absorbance at 800 nm showed the highest value, the absorbance at 800 nm was measured as the turbidity that substantially reflected light-scattering by the swollen starch granules, corresponding to the dispersion state of the swollen starch granules. The turbidity in this study is expressed as the difference [Δ absorbance at 800 nm = (the absorbance of the 0.1 M Lys-suspension)- (that of the control)] between the absorbance of the gelatinized suspension containing 0.1 M Lys and that of the control suspension (Fig. 5) . The Δ absorbance value below 60°C was negative due to the reduced swelling with Lys as already described. However, contrary to this, the value was positive above 70°C from increased light-scattering, probably due to formation of aggregates consisting of some swollen starch granules. The 70°C-gelatinized Act-WS suspension containing 0.1 M Lys and the control suspension were thus observed by a polarizing microscope. The presence of Lys induced an increase in the ratio of the aggregates mainly consisting of 2‒3 swollen granules (Fig. 6) . Measurement of granular size distribution showed that the median diameter of the gelatinized Act-WS suspension containing 0.1 M Gly was almost the same as that in distilled water at 60‒100°C, whereas the median diameter of the suspension containing 0.1 M Lys was significantly larger than those with 0.1 M Gly and in distilled water due to a decreased content of swollen granules below 60 μm and an increase in that above 60 μm (Fig. 7) . These results indicate that the characteristic increase in viscosity of the wheat starch paste with Lys would have been due to formation of aggregates consisting of multiple swollen granules during heating. Such aggregation of the swollen starch granules may have been induced through some interaction between Lys and a starch-granule-associated protein like friabilin.6) However, the possible aggregation mechanism for the swollen wheat starch granules with Lys needs to be investigated in a further study.
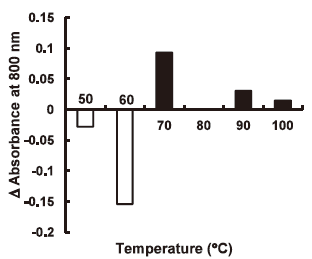
Effect of Lys on the turbidity of the 0.1% Act-WS suspension heated at 50, 60, 70, 90 and 100°C for 20 min while stirring at 160 rpm.
Turbidity is expressed as the difference (Δ absorbance at 800 nm) between the absorbance at 800 nm of the gelatinized suspension containing 0.1 M Lys and that of the control suspension (the absorbance of 0.1 M Lys-containing suspension‒that of the control suspension).

Distribution of multiple granules in the gelatinized Act-WS suspension of 0.1 M Lys or without Lys (Control) evaluated by microscopic observation.
The 0.1% Act-WS suspension was heated at 70°C for 20 min while stirring at 160 rpm, and then stained with iodine before microscopic observation. The distribution (%) was obtained by observing ten visible fields randomly selected. Single, single granule; 2‒3, aggregates consisting of 2‒3 granules; 4‒5, aggregates consisting of 4‒5 granules; 6 <, aggregates consisting of 6 or more granules.

Median diameter of the gelatinized Act-WS suspension of 0.1 M Gly, 0.1 M Lys or without Lys (Control) evaluated by a laser diffraction particle size analyzer.
The 0.1% Act-WS suspension was heated at 70, 90 and 100°C for 20 min while stirring at 160 rpm, and then sonicated for 30 s. The resulting suspension was diluted until the absorbance at 500 nm had reached within 0.1‒0.2 before measuring the distribution of the particle size.  , control without an amino acid;
, control without an amino acid;  , Gly;
, Gly;  , Lys. Different letters show significant difference (p < 0.05).
, Lys. Different letters show significant difference (p < 0.05).
In the present study, wheat starch exhibited the characteristic thermal behavior in terms of the significantly increased gelatinization temperature and viscosity with adding GluNa and Lys, in spite of the significantly reduced swelling and solubility. This increased viscosity of wheat starch with Lys contrasted the previous results1) 3) 4) 5) for potato starch. Since Lys elevated the turbidity, median diameter and ratio of aggregates of the swollen granules during heating, the increased viscosity was caused by forming aggregates with Lys. It is expected that inhibition of collapse of the swollen starch granules with adding charged amino acids could achieve easy water vaporization of the external water of swollen starch granules in wheat flower batter, contributing to improving the crispy texture of starch-dressed foods.
We are grateful to Associate Professor T. Kondoh of Kyoritsu Women’s University for measuring RVA, Dr. H. Takahashi of Ensuiko Sugar Refining Co. for measuring the granular size distribution, Dr. T. Yagishita of Nissin Flour Milling for providing soft wheat flour and Dr. N. Nio of Ajinomoto Co. for providing amino acids, respectively.