2013 Volume 60 Issue 2 Pages 137-139
2013 Volume 60 Issue 2 Pages 137-139
We developed an enzymatic colorimetric method for the quantification of α-D-mannose 1-phosphate by adding phosphomannomutase, mannose 6-phosphate isomerase and glucose 6-phosphate isomerase to a conventional glucose 6-phosphate assay using glucose 6-phosphate dehydrogenase. In this method, α-D-mannose 1-phosphate is converted into D-glucose 6-phosphate via D-mannose 6-phosphate and D-fructose 6-phosphate and the resultant D-glucose 6-phosphate is ultimately converted into 6-phosphogluconolactone under concomitant reduction of thio-NAD+ to thio-NADH, which can be quantified by its wavelength of 400 nm. This method is not altered by the presence of D-mannose, D-mannosamine, N-acetyl-D-mannosamine, L-mannose, β-1,4-mannobiose, α-1,2-mannobiose, methyl α-D-mannoside or dimethyl sulfoxide and it would be useful in studies involving enzymes such as phosphorylases belonging to glycoside hydrolase family 130, which release α-D-mannose 1-phosphate as the reaction product.
α-D-Mannose 1-phosphate (α-Man1P) is an essential intermediate in the biosynthesis of mannosides in polysaccharides, glycoproteins and glycolipids (Fig. 1). 1) In this biosynthesis, α-Man1P is generated from D-mannose 6-phosphate (Man6P) by phosphomannomutase (PMM, EC 5.4.2.8) and converted into GDP-mannose (GDP-Man) by mannose 1-phosphate guanylyltransferase (EC 2.7.7.13) to be the donor substrate for various mannosyltransferases such as β-1,4-mannosyltransferase (EC 2.4.1.142)2) and dolichol-phosphate mannosyltransferase (EC 2.4.1.83)3) . In addition, bacterial β-1,4-mannan-catabolic pathway via α-Man1P has been recently described (Fig. 1). In this pathway, β-1,4-mannan is hydrolyzed by extracellular mannanase into β-1,4-mannooligosaccharides (Mann: subscripted n means degree of polymerization), and the resultant Manns are incorporated into the cell. Mann (n ≥ 3) is phosphorolyzed into α-Man1P and Man(n-1) by β-1,4-D-mannooligosaccharide phosphorylase (EC 2.4.1.-).4) Man2 is converted into 4-O-β-D-mannosyl-D-glucose (ManGlc) by cellobiose 2-epimerase (EC 5.1.3.11), followed by phosphorolysis into α-Man1P and D-glucose by 4-O-β-D-mannosyl-D-glucose phosphorylase (EC 2.4.1.281).5) The resultant α-Man1P is converted into D-fructose 6-phosphate (Fru6P) by PMM and D-mannose 6-phosphate isomerase (M6PI, EC 5.3.1.8), and ultimately enters the glycolytic pathway.

Schematic diagram of the colorimetric quantification of α-Man1P referred with its biological role.
Bold arrows, the colorimetric quantification of α-Man1P; thin arrows, biosynthetic pathway of mannosides; dotted arrows, bacterial phosphorolytic pathway of β-1,4-mannan.
Because of the discovery of various physiological roles of α-Man1P, the demands for quantifying the compound have been increasing. Although α-Man1P can be generally measured quantitatively by using chromatographic techniques such as HPLC6) or by using fluorophore-assisted carbohydrate electrophoresis,7) these assays are often cumbersome, time-consuming and can process only a limited number of samples. Hence, alternative colorimetric methods for quantifying α-Man1P and those that can process a large number of samples are required.
To date, colorimetric methods for the quantification of various sugar 1-phosphates have been established based on the enzymatic quantification of D-glucose 6-phosphate (Glc6P) using glucose 6-phosphate dehydrogenase (G6PDH, EC 1.1.1.49) by measuring the increase in NAD(P)H spectrophotometrically at 340 nm. For the quantification of α-D-glucose 1-phosphate (α-Glc1P) and β-D-glucose 1-phosphate, they are converted into Glc6P by α- and β-phosphoglucomutases (EC 5.4.2.2 and EC 5.4.2.6), respectively, followed by the action of G6PDH.8) 9) 10) We previously developed an enzymatic colorimetric method for quantifying α-D-galactose 1-phosphate (α-Gal1P) by adding UDP-glucose hexose-1-phosphate uridylyltransferase (EC 2.7.7.12) and UDP-glucose to the α-Glc1P assay. 11) This quantification has contributed to the characterization of the glycoside hydrolase family (GH) 12) 112 β-D-galactoside phosphorylases that generate α-Gal1P. 11) 13) 14)
In this study, we describe a colorimetric method for quantifying α-Man1P. This method involves the addition of PMM, M6PI and glucose 6-phosphate isomerase (G6PI, EC 5.3.1.9) to the reagents of the Glc6P assay containing G6PDH (Fig. 1). We employed thio-NAD+, substituting NAD+ for the measurement at visible wavelength.
α-Man1P bis(cyclohexylammonium) salt was purchased from Sigma-Aldrich Corporation (St. Louis, USA). The recombinant G6PDH from Leuconostoc mesenteroides and thio-NAD+ were obtained from Oriental Yeast (Tokyo, Japan). The PMM,15) M6PI16) and G6PI17) genes (GenBank ID, AAC75109, AAC74685 and AAC76995, respectively) from Escherichia coli K-12 MG1655 were amplified by PCR with KOD-plus DNA polymerase (Toyobo, Osaka, Japan) with the following oligonucleotides based on the genomic sequence (GenBank ID, U00096):18) 5′-gagatatacatatgaaaaaattaacctgc-3′, 5′-gagatatacatatgcaaaaactcattaac-3′ and 5′-gagatatacatatgaaaaacatcaatccaac-3′ as the forward primers containing an NdeI site (underlined) and 5′-tggtgctcgagctcgttcagcaacgtcagc-3′, 5′-tggtgctcgagcagcttgttgtaaacacgc-3′ and 5′-tggtgctcgagaccgcgccacgctttatag-3′ as the reverse primers containing an XhoI site (underlined), respectively. Each amplified gene was purified using a FastGene Gel/PCR Extraction Kit (Nippon Genetics Co., Ltd., Tokyo, Japan), digested by NdeI and XhoI (New England Biolabs, Beverly, USA), and inserted into pET24a (+) (Novagen Inc., Madison, USA) to add a His6-tag at the C-terminal of the recombinant protein. The expression plasmids were propagated in E. coli DH5α, purified by a FastGene Plasmid Mini Kit (Nippon Genetics Co., Ltd.), and verified by sequencing (Operon Biotechnologies, Tokyo, Japan). E. coli BL21 (DE3) (Novagen Inc.) transformants harboring each expression plasmid were grown at 37°C in 200 mL Luria-Bertani medium (1% tryptone, 0.5% yeast extract and 0.5% NaCl) containing 50 μg/mL of kanamycin up to an absorbance of 0.6 at 600 nm. Expression was induced by 0.1 mM isopropyl β-D-thiogalactopyranoside and continued at 18°C for 24 h. Wet cells, harvested by centrifugation at 20,000 × G for 20 min, were suspended in 50 mM HEPES-NaOH buffer (pH 7.0) containing 500 mM NaCl (buffer A). The suspended cells were disrupted by sonication (Branson sonifier 250A; Branson Ultrasonics Co., Danbury, USA) and the supernatant, collected by centrifugation at 20,000 × G for 20 min, was applied to a HisTrap HP column (0.7 cm internal diameter × 2.5 cm, GE Healthcare, Buckinghamshire, UK) equilibrated with buffer A containing 10 mM imidazole using an ÄKTA prime (GE Healthcare). After a wash with buffer A containing 22 mM imidazole and following elution using a 22–400 mM imidazole linear gradient in buffer A, fractions containing recombinant protein were pooled, dialyzed against 10 mM HEPES-NaOH buffer (pH 7.0), and concentrated using an AMICON Ultra-15 filter (Millipore Co., Billerica, USA). The protein concentrations of PMM, M6PI and G6PI were determined spectrophotometrically at 280 nm using a theoretical extinction coefficient of E0.1% = 0.774, 0.738 and 1.26, respectively, based on the each amino acid sequence.19) The amounts of purified PMM, M6PI and G6PI obtained from cell lysate of 200 mL cultures were approximately 50, 138 and 209 mg, respectively. All other chemicals used in this study were reagent grade.
The working reagent for the α-Man1P assay was prepared by mixing 1 mM thio-NAD+, 0.40 mg/mL PMM, 0.55 mg/mL M6PI, 0.45 mg/mL G6PI and 0.012 mg/mL G6PDH in 50 mM MOPS-NaOH buffer (pH 7.0) containing 5 mM MgCl2. An aliquot (40 μL) of solution containing 0 to 0.8 mM α-Man1P was mixed with 40 μL of the working reagent in a well of a 96-well microtiter plate (GDMP-96F, As One Co., Osaka, Japan), followed by incubation at 37°C for 60 min. The concentration of α-Man1P released was measured by determining the absorbance at 400 nm using a temperature-controlled microplate reader (Multiscan Go, Thermo Fisher Scientific, Inc., Waltham, USA).
The time course of α-Man1P generated using the above protocol was monitored at 37°C by using a temperature-controlled microplate reader with the light path of 2.10 mm (Fig. 2A). The reaction was complete after 30 min and the absorbance did not decrease substantially thereafter up to 60 min. Therefore, we selected a reaction time of 60 min for this protocol. When we measured the relationship between the concentration of α-Man1P and the absorbance at 400 nm with triplicate measurements at each concentration of α-Man1P, the α-Man1P calibration curve was linear in the concentration range from 0 to 0.8 mM (Fig. 2B). The following relationship was derived from linear regression with a correlation coefficient greater than 0.999.
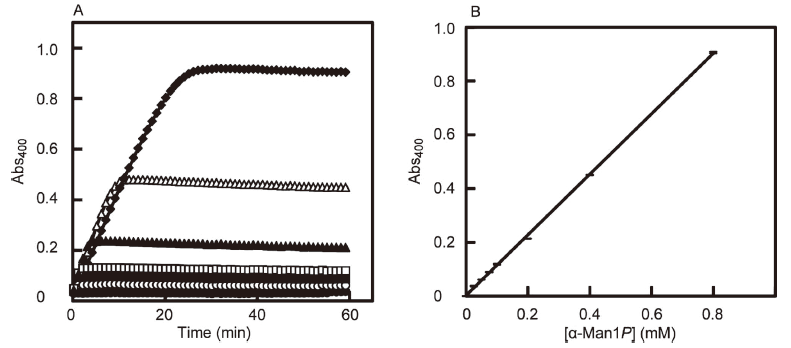
Time course and calibration curve for the quantification of α-Man1P.
(A) Time course of color development during the quantification of α-Man1P using thio-NAD+. The concentrations of α-Man1P were 0.025 mM (closed circles), 0.050 mM (open circles), 0.075 mM (closed squares), 0.1 mM (open squares), 0.2 mM (closed triangles), 0.4 mM (open triangles) and 0.8 mM (closed diamonds). (B) Calibration curve of α-Man1P using thio-NAD+. The measurements were performed in triplicate, and the standard deviations are indicated with error bars.
y = 1.13x + 0.0025 (x = [α-Man1P] (mM); y = Abs400)
The α-Man1P assay was not altered by the presence of 0.5 mM D-mannose, D-mannosamine, N-acetyl-D-mannosamine, L-mannose, β-1,4-mannobiose, α-1,2-mannobiose, methyl α-D-mannoside or 3.2 M dimethyl sulfoxide. It should be noted that (thio-)NAD+ must be substituted with (thio-)NADP+ when using G6PDH from yeast instead of one from bacteria, because of the specificity.20)
In conclusion, we developed an enzymatic colorimetric method for the quantification of α-Man1P by adding PMM, M6PI and G6PI to a conventional Glc6P assay using G6PDH. This method would be useful in studies of enzymes such as phosphorylases belonging to GH 130, which release the α-Man1P as the reaction product.
This work was supported in part by MEXT’s program “Promotion of Environmental Improvement for Independence of Young Researchers” under the Special Coordination Funds for Promoting Science and Technology.