2015 Volume 62 Issue 3 Pages 101-106
2015 Volume 62 Issue 3 Pages 101-106
We reported previously that oral administration of high molecular weight proteoglycan (molecular weight ≥ 5,000,000) from salmon nasal cartilage showed excellent effects on the skin and joints. We have developed a new method for extraction of high molecular weight proteoglycan at high yield and low cost for utilization in food. Examination of the extraction method involving heating of shredded salmon nasal cartilage pieces dispersed in water indicated that high molecular weight proteoglycan (molecular weight ≥ 5,000,000) was extracted efficiently by controlling the heating temperature and time based on the molten state of cartilage pieces. Three proteoglycan fractions of different molecular weights from salmon nasal cartilage were purified from the hot water extract under optimal condition. Quantification of hyaluronic acid and collagen showed that the proteoglycan fraction with molecular weight ≥ 5,000,000 consisted of hyaluronic acid-collagen-proteoglycan complex. The hot water extraction method established in this study can be used to extract the hyaluronic acid-collagen-proteoglycan complex from salmon nasal cartilage, and we have designated the salmon nasal cartilage extract obtained by this process as “Hyaluco PG”. The only solvent necessary for the hot water extraction method is water and the process is simple, both of which can drastically reduce costs compared to processes using acid, alkali, enzymes, or organic solvents such as ethanol. The hot water extraction method will allow the development of novel food materials with beneficial effects on health and beauty.
Proteoglycan (PG) is macromolecule consisting of a core protein with covalently linked glycosaminoglycan chains,1) and it is a major component of cartilage.2) In cartilage tissue, large numbers of PG molecules are bound noncovalently to the axis of hyaluronic acid, and forms huge aggregates.3) 4) 5) In addition, hyaluronic acid-PG aggregates form a network structure with collagen; they play roles as a cushion to maintain the water retention capacity and soften the physical stimulation of tissue.6) 7) 8)
The effects of orally administered collagen and hyaluronic acid have been studied,9) 10) 11) 12) and they have both been widely used as foods/supplements for beauty and health. On the other hand, although PG has expected to have excellent physiological activities, the difficulty of extraction has prevented applied research and practical study of these molecules. Takagaki et al. developed a low cost technique for extraction of PG from salmon nasal cartilage using acetic acid,13) and this method has been used as a source of material for PG research. This has promoted structural analysis, bioactivity studies, and applied research of salmon nasal cartilage PG. Kakizaki et al. reported the detailed structure of salmon nasal cartilage PG based on analysis of the amino acid sequence of core protein and analysis of sugar chains.14) Basis of this report, salmon nasal cartilage PG was estimated to have a molecular weight of about 1,800,000. In bioactivity studies, Sashinami et al. reported that salmon nasal cartilage PG modulates the cytokine response of murine macrophages to Escherichia coli.15) Several studies have shown that oral administration of salmon nasal cartilage PG suppresses the progression of colitis.16) 17) In addition, Asano et al. reported that oral administration of commercially available salmon nasal cartilage PG was associated with improvement in the mouse intestinal flora.18) However, the molecular weight and structure of salmon nasal cartilage PG used in these bioactivity studies were not clear.
To study the relationship between molecular weight and biological activities of PG, we purified the native form of PG from the water extract of defatted powdered salmon nasal cartilage19) by ion exchange chromatography. The samples were subsequently divided into three fractions of different molecular weights from the native form PG by gel filtration chromatography. The anti-aging effects of oral administration of these three different molecular weight PG fractions on skin were investigated using skin aging model hairless mice, and we reported that largest molecular weight PG fraction (molecular weight ≥ 5,000,000) showed the greatest efficacy against photoaging of the skin.20) Furthermore, in an oral administration test in osteoarthritis model mice, we compared the effects of high molecular weight PG (molecular weight ≥ 1,800,000) and low molecular weight PG (molecular weight < 1,800,000). The results indicated that only the oral administration of high molecular weight PG significantly inhibited damage to cartilage in these model mice.21) These observations suggested that high molecular weight PG may be useful materials for enhancement of beauty and health in the aging society. However, currently available commercial PG is acetic acid extract; reduction of molecular weight by acid extraction has been confirmed.22) Therefore, the present study was performed to develop a novel method for the efficient extraction of high molecular weight PG.
Kakizaki et al. confirmed that salmon nasal cartilage PG from 4 M guanidine hydrochloride extract retains the native PG structure by atomic force microscopy,22) but guanidine hydrochloride cannot be used in the production of food materials. Water extraction from defatted powdered salmon nasal cartilage can extract high molecular weight PG.23) However, this method was difficult to scale up for industrial production due the low yield of PG and the complicated defatted powderization process. We confirmed that the extraction efficiency of PG can be increased by raising the temperature of the water extraction step from defatted powdered salmon nasal cartilage. Conventionally, PG was thought to be denatured or decomposed by heat. However, the impact of heating on the structure of PG in cartilage has not been investigated in detail. In this study, we examined the effects of heating of PG present in tissues of salmon nasal cartilage, and developed a novel method involving direct heating of cartilage dispersed in water for efficient extraction of natural type high molecular weight PG.
Materials. Frozen salmon nasal cartilages were purchased from a local agency (Aomori, Japan). All chemicals used in each buffer were of analytical grade.
Quantity of glucuronic acid. The sugar chains of cartilage PG are predominantly chondroitin sulfate,24) which is a repeated sequence of D-glucuronic acid and N-acetyl-D-galactosamine bound by sulfuric acid. Therefore, it is possible to estimate the amount of PG by measuring the amount of glucuronic acid. The glucuronic acid levels of PG were quantified using the carbazole-sulfuric acid method.25)
PG extraction from salmon nasal cartilage. Cartilages of frozen salmon were shredded in a blender (Waring J-SPEC Blender 700BUJ; Waring Products, Inc., Stamford, CT). The pieces of shredded salmon nasal cartilage were suspended in 2.5 volumes of distilled water, and stirred for various times (2‒6 h) at various temperatures (50‒90°C). After heating, the molten states of the cartilage pieces were examined. Then, samples were centrifuged for 30 min at 12,000 × G, 4°C. After centrifugation, the supernatants, which were removed a fat layer at the top, were filtered with suction using laboratory filter paper (Qualitative filter paper No. 1; Advantec Toyo Kaisha, Ltd., Tokyo, Japan). The filtered supernatants were then freeze-dried to obtain the powdered hot water extracts. For measurement of PG extraction efficiency, the recovery amounts and glucuronic acid amounts of the powdered hot water extracts were measured, and the amounts of glucuronic acid per 100 g of salmon nasal cartilage were calculated.
Molecular weight distribution measurement of PG by gel filtration column chromatography. As molecular weight markers, three dextrans of different molecular weights were prepared as follows: dextran from Leuconostoc mesenteroides (average Mw 5,000,000‒40,000,000; Sigma-Aldrich Corp., St. Louis, USA); (D1) dextran standard 1,400,000 from L. mesenteroides (MW 1,400,000; Sigma-Aldrich) (D2); and dextran standard 410,000 from L. mesenteroides (Mw 410,000; Sigma-Aldrich) (D3). Powdered hot water extracts from salmon nasal cartilage equivalent to 1 mg of glucuronic acid were dissolved in 1 mL of 0.1 M phosphate buffer (pH 7.1) containing 0.2 M sodium chloride (0.1 M phosphate buffer), applied to a Sepharose CL-2B gel filtration column (φ1.0 × 50 cm; GE Healthcare Japan Co., Tokyo, Japan) equilibrated with 0.1 M phosphate buffer, and then eluted with the same buffer. Dextran markers (D1, D2, D3; 0.5 mg each) were dissolved in 1 mL of 0.1 M phosphate buffer, subjected to Sepharose CL-2B gel filtration column chromatography as described above at 4°C, and 1-mL fractions were collected. Next, amounts of the dextran of each dextran marker fractions were determined using the phenol-sulfuric acid method.26) As the optimal molecular weight separation range of Sepharose CL-2B for dextran is 100,000‒20,000,000, the molecular weight corresponding to the void volume (Vo) is 20,000,000; D1 was used to measure the void volume (Vo). A calibration curve of dextran molecular weight against fraction number was plotted, and the elution position of molecular weights 5,000,000, 1,800,000, and 400,000 were calculated. The amounts of glucuronic acid in each fractions of hot water extracts from salmon nasal cartilage were then determined using the carbazole-sulfuric acid method.
Quantification of hyaluronic acid and collagen. Sample of 50 mg of salmon nasal cartilage hot water extract (extraction temperature, 90°C; extraction time, 3 h) was dissolved in 20 mL of 7 M urea, 50 mM Tris-HCl, pH 7.4 (7 M urea buffer), and applied to a DEAE-Sephacel ion-exchange column (φ2.5 × 15 cm; GE Healthcare Japan), equilibrated with 7 M urea buffer and then eluted with a linear gradient of 0‒0.75 M NaCl in the same solvent. DEAE-Sephacel ion-exchange column chromatography was carried out at 4°C, and 16-mL fractions were collected. The amounts of glucuronic acid in each fraction were determined by the carbazole sulfate method. The fractions positive for glucuronic acid and with an elution position corresponding to 0.35‒0.5 M NaCl were regarded as PG and were pooled. Then, the PG fraction was desalted by dialysis (Spectra/Por® Biotech Cellulose Ester Dialysis Membranes, MWCO: 1,000,000; Spectrum Laboratories, Inc., Rancho Dominguez, USA), and dried by lyophilization. PG purified by ion exchange column chromatography was subjected to gel filtration elution column chromatography according to the procedure described above; note that 25 mg of PG fraction dissolved in 20 mL of 0.1 M phosphate buffer was eluted and subjected to Sepharose CL-2B gel filtration column (φ2.5 × 50 cm; GE Healthcare Japan), and 16-mL fractions were collected. Three different molecular weight fractions from those positive for glucuronic acid were collected: ≥ 5,000,000 (PG-1), ~1,800,000 (PG-2), and ~400,000 (PG-3). These fractions were desalted by dialysis, and dried by lyophilization. PG-1, 2, and 3 were examined for hyaluronic acid and collagen amounts using a Hyaluronic acid ELISA kit (QnE Hyaluronic Acid (HA) ELISA Assay; Biotech Trading Partners, Encinitas, USA) and a Collagen assay kit (Sircol Soluble COLLAGEN Assay; Biocolor Ltd., County Antrim, UK). To confirm the simple interaction of PG and collagen, acid-soluble collagen with no intermolecular crosslinking was quantified using the Collagen assay kit.
Molten state of salmon nasal cartilage and PG extraction efficiency by hot water extraction.
Table 1 shows the molten states of salmon nasal cartilage pieces and amounts of extracted glucuronic acid from aqueous dispersions of salmon nasal cartilage pieces, reflecting PG extraction efficiency, under different extraction conditions. Salmon nasal cartilage pieces were partially melted at 80°C for 6 h or 90°C for 2 h, and completely melted at 90°C for 3 h. The amounts of extracted glucuronic acid were significantly increased by melting of the cartilage pieces. The total amount of glucuronic acid present in the salmon nasal cartilage was 1.57 g as described by Kato et al.27) Therefore, the amount of extracted glucuronic acid from 100 g of salmon nasal cartilage at 90°C for 3 h was 1.38 g (Table 1), representing a very high extraction efficiency (88c). The amount of extracted glucuronic acid from 100 g of defatted powdered salmon nasal cartilage in water at room temperature for 4 h was 0.17 g (data not shown), indicating a low extraction efficiency (11%).
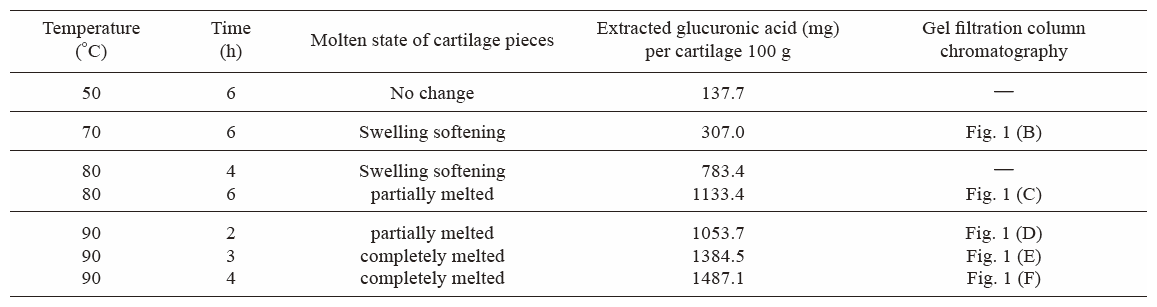
Molten states of salmon nasal cartilage pieces and amounts of extracted glucuronic acid from salmon nasal cartilage pieces under different extraction conditions.
Molecular weight distribution of salmon nasal cartilage PG in the hot water extraction method.
The results of CL-2B gel filtration column chromatography of salmon nasal cartilage extracts under different conditions are shown in Fig. 1. Figure 1 (A) shows the molecular weight distribution of PG from water extracts of defatted powdered salmon nasal cartilage at room temperature as a control, while (B)‒(F) show those of PG from hot water extract of salmon nasal cartilage with different temperatures and times. The process for manufacturing of the defatted powdered salmon nasal cartilage used as a control included ethanol treatment for defatting, which does not affect the quality of the proteoglycans. On the other hand, hot water extraction allows sufficient defatting of salmon nasal cartilage by centrifugation after the cartilage is dissolved by heating without using ethanol. PG with molecular weight ≥ 5,000,000 was abundant in water extracts of defatted powdered salmon nasal cartilage at room temperature (Fig. 1 (A)). At hot water extraction temperature of 50°C and 70°C, the peaks of molecular weight ≥ 5,000,000 were obviously lower than the control (Fig. 1 (A)). Therefore, high molecular weight PG (Mw, ≥ 5,000,000) was not sufficiently extracted under these conditions (50°C, data not shown; 70°C, Fig. 1 (B)). When the temperature was further increased, extractions at 80°C for 6 h (Fig. 1 (C)), 90°C for 2 h (Fig. 1 (D)), and 90°C for 3 h (Fig. 1 (E)) yielded abundant high molecular weight PG (MW, ≥ 5,000,000); these molecular weight distributions were similar to that shown in Fig. 1 (A). However, with further heating at 90°C for 4 h, the peak of the high molecular weight PG (MW, ≥ 5,000,000) was decreased (Fig. 1 (F)). The results of this study, indicated that PG with high molecular weight ≥ 5,000,000 from salmon nasal cartilage could be extracted efficiently by appropriately controlling the temperature and time in the molten state of salmon nasal cartilage.

CL-2B gel filtration column chromatography of salmon nasal cartilage extracts under different conditions.
(A), the molecular weight distribution of PG from water extracts of defatted powdered salmon nasal cartilage at room temperature for 4 h; (B)‒(F), the molecular weight distributions of PG from hot water extract of salmon nasal cartilage with different temperatures and times ((B), 70°C for 6 h; (C), 80°C for 6 h; (D), 90°C for 2 h; (E), 90°C for 3 h; (F), 90°C for 4 h).
Quantification of hyaluronic acid and acid-soluble collagen in three PG fractions from hot water extracts of salmon nasal cartilage.
Kakizaki et al. reported that the positive fractions of carbazole-sulfuric acid method in gel filtration column chromatography from 4 M guanidine hydrochloride extract of salmon nasal cartilage have the molecular structures of the PG based on atomic force microscope.22) Salmon nasal cartilage PG was estimated to have a molecular weight of about 1,800,000.14) Therefore, we assumed that the peak around the molecular weight of 1,800,000 was pure PG, and that with a molecular weight ≥ 5,000,000 consisted of PG-related complexes (Fig. 1). In cartilage tissue, PG molecules bind noncovalently to the axis of hyaluronic acid, and hyaluronic acid-PG aggregates form network structures with collagen.6) 7) 8) Because we considered that the hot water extraction method extracted PG complexes in intact form, we quantified hyaluronic acid and collagen of three PG fractions. As described in the Materials and Methods, three PG fractions of different molecular weights (PG-1, 2, and 3) were obtained from hot water extract of salmon nasal cartilage (extraction temperature, 90°C; extraction time, 3 h) by ion-exchange column chromatography and gel filtration column chromatography. The elution profile of hot water extract of salmon nasal cartilage on ion-exchange column chromatography is shown in Fig. 2, and the elution profile of purified PG (show in the bar of Fig. 2) on gel filtration column chromatography is shown in Fig. 3. On gel filtration column chromatography of the PG obtained by ion exchange column chromatography (Fig. 3), the peak with molecular weight ≥ 5,000,000 (PG-1) was clearly lower than that of PG-1 in Fig. 1 (E). Urea is used as a solubilizing agent in ion-exchange column chromatography to prevent aggregation by adsorption of insoluble proteins on DEAE. Therefore, the urea is likely to dissociate part of the PG complex. The three different molecular weight purified PG fractions (PG-1, 2, and 3) were examined for hyaluronic acid and acid-soluble collagen amounts (Table 2). Hyaluronic acid was most abundant in PG-1, which was dependent on the molecular weight. Hyaluronic acid forms large aggregates even when present in small amounts as many PG molecules bind noncovalently to one molecule of hyaluronic acid. Acid-soluble collagen, which is immature collagen that is not fibrous, was most abundant in PG-1, with almost none present in PG-2 and PG-3. As acid-soluble collagen was present specifically in PG-1, collagen was suggested to form bonds with hyaluronic acid-PG aggregates. These results indicated that the high molecular weight PG (Mw, ≥ 5,000,000) specifically contained hyaluronic acid-collagen-PG complex. In this process, it was assumed that the hyaluronic acid-collagen-PG complex in cartilage tissue was extracted without being degraded by melting of salmon nasal cartilage pieces dispersed in hot water. We designated the PG obtained from salmon nasal cartilage by hot water extraction as “Hyaluco PG”. The total PG content in Hyaluco PG was 45‒50% based on the results of the carbazole sulfuric acid method. The hyaluronic acid-collagen-PG complex content in the total PG was estimated to be 30‒40% based on calculation of the proportion of molecular weight ≥ 5,000,000 PG in the molecular weight distributions of the PG in Fig. 1 (C), (D), and (E).

DEAE-Sephacel ion-exchange column chromatography of hot water extract of salmon nasal cartilage.

Sepharose CL-2B gel filtration column chromatography of the PG fractions.
PG-1, PG (molecular weight ≥ 5,000,000); PG-2, PG (molecular weight ~1,800,000); PG-3, PG (molecular weight ~400,000).
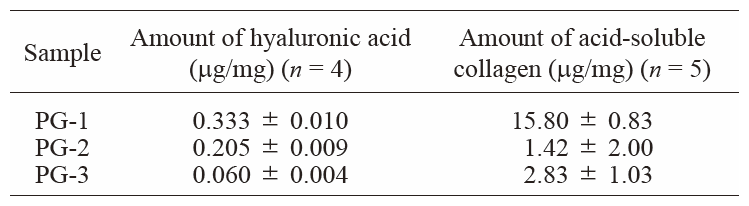
Quantification of hyaluronic acid and acid-soluble collagen in three PG fractions from hot water extracts of salmon nasal cartilage.
Values are the means ± SEM. PG-1, PG (molecular weight ≥ 5,000,000); PG-2, PG (molecular weight ~1,800,000); PG-3, PG (molecular weight ~400,000).
Oral administration of high molecular weight ≥ 5,000,000 PG to hairless mice was shown previously to have anti-inflammatory effects.20) Hyaluco PG may be effective against a variety of symptoms of inflammation in addition to prevention of skin aging and prevention/improvement of arthritis. The hot water extraction method uses only water as the solvent, which will allow a very simple manufacturing process at low cost compared with conventional PG. Hyaluco PG can be incorporated at effective amounts into food products by low-cost manufacturing, which will facilitate the development of food products with a variety of features using Hyaluco PG for improvement of both health and beauty.
This study was supported in part by the Regional Innovation Strategy Support Program (City Area Type) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.