2015 年 62 巻 4 号 p. 135-141
2015 年 62 巻 4 号 p. 135-141
We achieved to synthesize a novel inclusion supramolecular polymer composed of continuum of amylose-poly(tetrahydrofuran) (PTHF) inclusion complexes by phosphorylase-catalyzed enzymatic polymerization using a maltoheptaose-PTHF conjugate according vine-twining polymerization manner. The 1H NMR and X-ray diffraction measurements indicated the presence of the inclusion complex structure in the product. The GPC peak of the amylose segment, which was dissociated by heating the vine-twining polymerization product, shifted to the lower molecular weight region, compared with that of the product, supporting the structure of the inclusion supramolecular polymer. The product by the G-1-P/G7-PTHF feed ratio = 100 was the supramolecular polymer composed of continuum of the amylose-PTHF inclusion complexes, whereas both inclusion complexes and amylose double helixes were present in the product by the G-1-P/G7-PTHF feed ratio over 200.
G, D-glucose; G-1-P, α-D-glucose 1-phosphate; G7, maltoheptaose; PLLA, poly(L-lactide); PTHF, poly(tetrahydrofuran); NMR, nuclear magnetic resonance; XRD, X-ray diffraction; GPC, gel permeation chromatography; DMSO, dimethylsulfoxide, DP; degree of polymerization, MeOTf; methyl trifluoromethanesulfonate.
Amylose, a natural linear polysaccharide with left-handed helical conformation composed of D-glucose (G) residues through α(1→4) glycosidic linkages, is one component of starch,1) which has been extensively studied in the field of material research because of its low cost, biodegradability, and renewability. It is a well-known host molecule that readily forms inclusion complexes with various low molecular-weight guest compounds by hydrophobic interaction between guest molecules and the cavity of its helix.2) 3) 4) 5) 6) 7) 8) However, little has been reported regarding the formation of inclusion complex composed of amylose and polymeric compounds.9) 10) 11) 12) 13) Loos et al. recently reported a facile preparation method for inclusion complexes composed of amylose and synthetic polymer via direct mixing at high temperature.14) 15) 16) 17) The principal difficulty for incorporating polymeric materials into the cavity of amylose is that the driving force for binding is only due to weak hydrophobic interaction. Amylose, therefore, does not have the sufficient ability to include the long chains of polymeric guests into its cavity.
By means of the enzymatic method for direct construction of polysaccharides,18) 19) 20) 21) 22) 23) we have developed the new methodology for the preparation of inclusion complexes composed of amylose and synthetic polymers,24) 25) 26) 27) 28) which was achieved by the phosphorylase-catalyzed enzymatic polymerization forming amylose in the presence of guest hydrophobic polymers dispersed in polymerization media. Phosphorylase-catalyzed enzymatic polymerization using α-D-glucose 1-phosphate (G-1-P) as a monomer proceeds with the regio- and stereoselective construction of an α-glycosidic bond under mild conditions, leading to the direct formation of amylose in aqueous media.29) 30) 31) 32) 33) 34) This polymerization is initiated from a maltooligosaccharide primer such as maltoheptaose (G7). The propagation proceeds through the reversible reaction to produce an α(1→4)-glucan chain, that is amylose, where the enzymatic catalysis transferred a glucose unit to the nonreducing 4-OH terminus of an α(1→4)-glucan chain from G-1-P and liberated an inorganic phosphate (Pi) as follows; [α(1→4)-G]n + G-1-P→[α(1→4)-G]n+1 + Pi.
The representation of the above enzymatic polymerization for the construction of such amylose-polymer inclusion complex is similar to the way that a vine of plant grows twining around a rod. Accordingly, we have proposed that this polymerization method is named “vine-twining polymerization”. As the guest polymers for this polymerization system, hydrophobic polyethers,35) 36) polyesters,37) 38) 39) 40) and polycarbonates41) have been used to form corresponding inclusion complexes with amylose. In addition, the vine-twining polymerization has been applied to selective inclusion by amylose toward polymers with particular structures, molecular weight distributions, and chiralities.42) 43) 44) 45)
We recently achieved to synthesize a novel supramolecular polymer, which was composed of continuum of the amylose-poly(L-lactide) (PLLA) inclusion complexes, was produced when a G7-functionalyzed PLLA was used as a primer-guest conjugate substrate in the vine-twining polymerization.46) 47) In this approach, a propagating amylose chain started from G7 moiety in the primer-guest conjugate by the phosphorylase catalysis includes a polyester, PLLA, segment in the other substrate. Such special type of the vine-twining polymerization among the substrates successively takes place, giving rise to the novel inclusion supramolecular polymer composed of amylose and PLLA. This vine-twining polymerization approach for the supramolecular polymer has inspired us to be applicable to other hydrophobic polymers. Because it was found to form the inclusion complex with amylose,35) 36) in this paper, we employed a polyether, poly(tetrahydrofuran) (PTHF), and demonstrated the synthesis of inclusion supramolecular polymer composed of amylose and PTHF (amylose-PTHF) by means of the vine-twining polymerization using G7-functionalized PTHF (G7-PTHF) as a primer-guest conjugate.
Materials. G7 was prepared by selective cleavage of one glycosidic bond of β-cyclodextrin under acidic conditions.48) Phosphorylase from Aquifex aeolicus VF5 was supplied from Ezaki Glico Co., Ltd., Osaka, Japan.32) 49) 50) Other reagents and solvents were available commercially and used without further purification.
Measurements. 1H NMR spectra were recorded on JEOL ECA 600 (JEOL Resonance Inc., Tokyo, Japan) and Bruker BioSpin AV-300 spectrometers (Bruker Corporation, Billerica, USA). XRD measurement was conducted using a PANalytical X’Pert Pro MPD (Yamato Scientific Co., Ltd., Tokyo, Japan) with Ni-filtered CuKα radiation (λ = 0.15418 nm). GPC analysis for PTHF was performed by using a HITACHI pump L-2130 and a HITACHI RI detector L-2490 (Hitachi High-Technologies Corporation, Tokyo, Japan) under the following conditions: Shodex GPC KF-804L and KF-803L (Showa Denko K.K., Tokyo, Japan) columns with chloroform as the eluent at a flow rate of 1.0 mL/min at 40°C using polystyrene samples as standards. GPC analysis for vine-twining product was performed by using a Shimadzu pump LC-9A (Shimadzu Corporation, Kyoto, Japan) and a Tosoh RI detector RI-8020 (Tosoh Corporation, Tokyo, Japan) under the following conditions; Shodex GPC LF-804 (8.0 × 300 mm) (Showa Denko K.K.) column with dimethyl sulfoxide (DMSO) as the eluent at a flow rate of 0.3 mL/min at 50°C using pullulan samples as standards. UV-Vis measurements were conducted using a Jasco V-650Q1 spectrometer (JASCO Corporation, Tokyo, Japan).
Synthesis of acetylene-terminated PTHF. Under argon, a mixture of THF (2.0 mL, 25 mmol) with methyl trifluoromethanesulfonate (MeOTf) (0.1 mL, 1.0 mmol) was stirred for 1 h at 0°C. Propargyl alkoxide was individually prepared by slowly mixing propargyl alcohol (4.0 mL, 5.0 mmol) with a suspension of sodium hydride (60% with mineral oil, 0.2 g, 5.0 mmol) in THF (2.0 mL). After the mixture was added to the polymerization solution, it was stirred overnight at room temperature. The resulting solution was diluted with water and extracted with dichloromethane. The organic layer was concentrated and dried under reduced pressure to give acetylene-terminated PTHF (1.35 g, functionality of acetylene group at the terminal end = 60%, Mn(NMR) = 1.6 × 103, Mn(GPC) = 7.1 × 103, Mw/Mn = 1.69).
1H NMR (600 MHz, CDCl3): δ (ppm) 4.13 (1H, O-CH2-C≡), 3.41 (OCH2 of PTHF), 3.33 (3H, OCH3), 2.42 (1H, ≡CH), 1.62 (C-CH2-C of PTHF).
Synthesis of G7-PTHF. To a 1,3-dioxolane solution (0.75 mL) of acetylene-terminated PTHF (80 mg, 0.05 mmol) was added a DMSO solution (0.25 mL) of β-maltoheptaoyl azide51) (G7-N3, 53 mg, 0.045 mmol), tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (2.7 mg, 0.005 mmol), and an aqueous solution (0.1 mL) of copper(II) sulfate pentahydrate (1.3 mg, 0.005 mmol) and L-ascorbic acid sodium salt (2.0 mg, 0.01 mmol). The resulting mixture was stirred for 24 h at 50°C, and purified after dialysis (MWCO 1000) and lyophilization to give G7-PTHF (78 mg, 68%).
1H NMR (300 MHz, DMSO-d6): δ (ppm) 8.23 (1H, s, triazole), 5.57 (1H, d, H1β of G, J1,2 = 8.7 Hz), 5.10-4.90 (6H, m, H1α of G), 4.63 (2H, C=C-CH2-O), 3.80-3.00 (H2-6 of G and OCH2 of PTHF), 3.10 (OCH3), 1.44 (C-CH2-C of PTHF).
Synthesis of amylose-PTHF by vine-twining polymerization. G7-PTHF (2.8 mg, 1.0 μmol) was suspended in sodium acetate buffer (4.0 mL, 0.2 mol/L, pH 6.2) by ultrasonic treatment. After the addition of G-1-P (30.4 mg, 0.1 mmol) and thermostable phosphorylase from Aquifex aeolicus VF5 (9 U), the mixture was stirred vigorously for 48 h at 40-45°C. The precipitated product was collected by filtration, washed with water, acetone, and chloroform, and then dried under reduced pressure at room temperature to give the supramolecular polymer product (amylose-PTHF, 13.6 mg).
Dissociation of host-guest inclusion of amylose-PTHF. A DMSO solution (2 mL) of amylose-PTHF (10 mg) was stirred for 24 h at 100°C. The resulting solution was dried under reduced pressure to give the dissociated materials.
In order to synthesize the amylose-PTHF in the vine-twining polymerization, we designed G7-PTHF as the primer-guest conjugate, and accordingly, synthesized it from G7-N3 and acetylene-terminated PTHF by copper(I)-catalyzed azide-alkyne cycloaddition, click chemistry52) (Fig. 1(a)); the former was prepared by the literature procedure51) and the latter was obtained by ring-opening polymerization of tetrahydrofuran by using MeOTf, followed by treatment with sodium propargyl alkoxide. The 1H NMR spectrum of the product in DMSO-d6 fully supported the structure of G7-PTHF (Fig. 2(a)).

(a) Synthesis of primer-guest conjugate (G7-PTHF) and (b) vine-twining polymerization using G7-PTHF to produce inclusion supramolecular polymer (amylose-PTHF).

1H NMR spectra of (a) G7-PTHF and (b) vine-twining polymerization product by G-1-P/G7-PTHF feed ratio of 100 (DMSO-d6).
The vine-twining polymerization using the resulting G7-PTHF was then conducted to synthesize amylose-PTHF as follows (Fig. 1(b)). Frist, G7-PTHF was suspended in sodium acetate buffer (pH 6.2) using an ultrasonic wave. After the addition of G-1-P (100 equiv. for G7-PTHF) and thermostable phosphorylase from Aquifex aeolicus VF5, the mixture was stirred vigorously for 48 h at 40-45°C. The precipitated product was collected by filtration, washed with water, acetone, and chloroform, and then dried under reduced pressure at room temperature to obtain the product.
The XRD pattern of the product (Fig. 3(a)) shows two diffraction peaks at 2θ = 13° and 20°, which is completely different from that of an enzymatically synthesized amylose (by the phosphorylase-catalyzed enzymatic polymerization), G7-PTHF, and PTHF (Figs. 3(c)-(e)), but is similar to that of the standard inclusion complex product from G7 and PTHF as prepared by our literature procedure (Fig. 3(b))35) 36). This result strongly suggests that the produced amylose formed inclusion complex with PTHF segment in the conjugate during the progress of the enzymatic polymerization. The 1H NMR spectrum of the product in DMSO-d6 (Fig. 2(b)) shows both signals at 5.1 and 3.7-3.1 ppm due to amylose (H1 and H2-6, respectively) and methylene signals of PTHF at 3.2 and 1.5 ppm. The intensity ratio of saccharide signals to the PTHF signals from the product was larger compared with that from G7-PTHF (Fig. 2(a)), indicating the success of the phosphorylase-catalyzed enzymatic polymerization. To estimate the included ratio of the PTHF chain to the amylose helix, furthermore, the integrated ratio of the methylene signal b to the H1 signal (b/H1) was calculated to be 0.84 in the case of G-1-P/G7-PTHF = 100, which was relatively close to the b/H1 value, 0.89, in the 1H NMR spectrum of the standard amylose-PTHF inclusion complex reported in our previous literature.36) This result suggested that the elongated amylose chain fully included PTHF segment.

XRD patterns of (a) vine-twining polymerization products from G-1-P/G7-PTHF feed ratio of 100, (b) amylose-PTHF inclusion complex from G7 and PTHF, (c) enzymatically synthesized amylose, (d) G7-PTHF, and (e) PTHF.
To dissociate the inclusion complexes, resulting in disintegration of the supramolecular polymeric structure, a DMSO solution of the vine-twining polymerization product was heated at 100°C and then dried under reduced pressure. The XRD pattern of the dissociated product (Fig. 4(b)) shows a diffraction peak at 2θ = 17° due to double helix of the amylose chains, as detected in that of the standard amylose (Fig. 4(c)), but did not observe diffraction peaks due to the inclusion complex as shown in that of amylose-PTHF (Fig. 4(a)). This result suggests that amylose chains completely released PTHF segments from the inclusion complexes in amylose-PTHF by the heating procedure in DMSO, which then constructed double helixes because amylose is known to spontaneously form double helix conformation.53) 54) The GPC peaks of the dissociated materials were detected at lower molecular weight region compared with those of the vine-twining polymerization product (Mn = 8.4 × 104, Mw/Mn = 1.63 → Mn = 1.7 × 104, Mw/Mn = 1.38 (Figs. 5(a) and (b)), supporting the supramolecular structure of the product formed by inclusion complexation of amylose with PTHF segments among the conjugates.

XRD patterns of (a) vine-twining polymerization products from G-1-P/G7-PTHF feed ratio of 100, (b) its dissociated materials by heating in DMSO, and (c) enzymatically synthesized amylose.

GPC charts of vine-twining polymerization products ((a), (c), and (e)) and their amylose segments ((b), (d), and (f)); G-1-P/G7-PTHF feed ratios of ((a) and (b)) 100, ((c) and (d)) 200, and ((e) and (f)) 300.
To evaluate the effect of G-1-P/G7-PTHF feed ratio on the formation of the supramolecular polymer, the vine-twining polymerization using G7-PTHF was performed in the higher G-1-P/G7-PTHF feed ratio, 200 and 300. The XRD patterns of the product (Figs. 6(a) and (b)) show not only the peaks at 13° and 20° due to inclusion complex as same as in that of the above amylose-PTHF (G-1-P/G7-PTHF feed ratio = 100, Fig. 6(c)), but also the peak at 17° due to double helix of the amylose chains, as detected in that of the standard amylose (Fig. 6(d)). This XRD result indicates that part of the elongated amylose chains did not include the PTHF segment, but formed double helix under the conditions of the higher G-1-P/G7-PTHF feed ratio. The b/H1 values were 0.55 and 0.31 respectively, suggesting that the presence of two kinds of amyloses in the products, either participates or does not participate into the inclusion with the PTHF segment. Indeed, the vine-twining polymerization products and their dissociated materials from higher G-1-P/G7-PTHF feed ratios had bimodal molecular weight distribution in the GPC results, due to the presence of two kinds of amyloses (Figs. 5(c)‒(f)). The GPC peaks of the dissociated materials eluted at later times were detected at lower molecular weight region compared with those of the vine-twining polymerization product (G-1-P/G7-PTHF feed ratio = 200; Mn = 9.9 × 104, Mw/Mn = 1.38 → Mn = 3.8 × 104, Mw/Mn = 1.29 (Figs. 5(c) and (d)). G-1-P/G7-PTHF feed ratio = 300; Mn = 1.8 × 105, Mw/Mn = 1.50 → Mn = 3.7 × 104, Mw/Mn = 1.53 (Figs. 5(e) and (f)). When the enzymatic polymerization was conducted in the higher G-1-P/G7 feed ratio, the chain propagation is probably faster than that in the lower feed ratio. Accordingly, some of the propagating amylose chains do not participate into the inclusion with the PTHF segment and continuously elongate to produce amylose with high molecular weight, which spontaneously forms double helix.
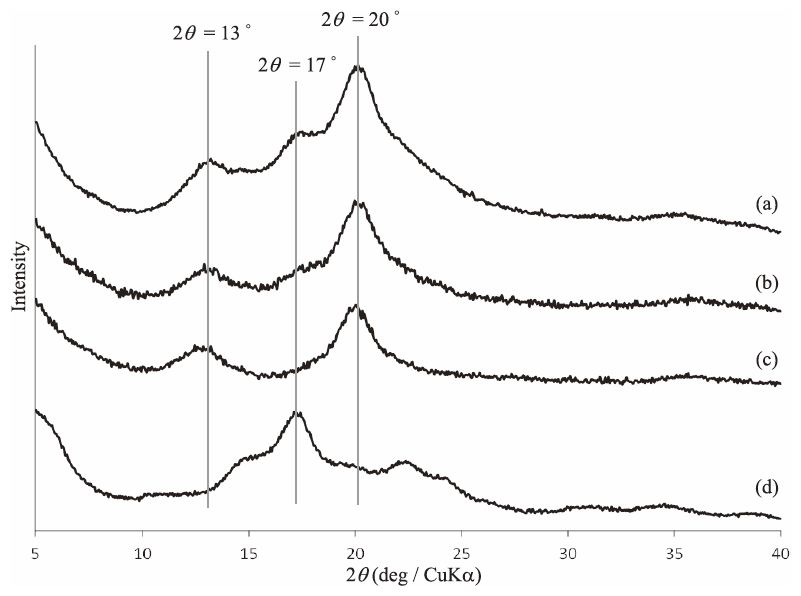
XRD patterns of vine-twining polymerization products by G-1-P/G7-PTHF feed ratios of (a) 300, (b) 200, and (c) 100, and (d) enzymatically synthesized amylose.
The DPs of amyloses in the dissociated materials were estimated by measuring the value of λmax in UV-vis spectrum after complexation with iodine,55) which further revealed the difference in their conformations formed in the vine-twining polymerization depending on G-1-P/G7-PTHF feed ratios. In the case of G-1-P/G7-PTHF feed ratio, 100, the DP of amylose chain was 97; Mn(amylose) = 15,700. On the other hand, the DPs of amylose chain were increased with increasing the G-1-P/G7-PTHF feed ratios, 200 and 300; the DPs of amylose chain were 118; Mn(amylose) = 19,200 and 151; Mn(amylose) = 24,500, respectively. These results indicated that the polymerization conditions in higher excess feed ratio of G-1-P produced amylose segment which did not participate into the inclusion with the PTHF segment, and further elongated to form a higher molecular weight chain with double helix conformation.
In conclusion, we achieved to synthesize the novel inclusion supramolecular polymer composed of amylose and PTHF by vine-twining polymerization using a primer-guest polymer conjugate, G7-PTHF. The supramolecular polymer, amylose-PTHF, was composed a polymeric continuum of some amylose moieties included PTHF moieties. The product by the G-1-P/G7-PTHF feed ratio = 100 was the supramolecular polymer composed of continuum of the amylose-PTHF inclusion complexes, whereas both inclusion complexes and amylose double helixes were present in the product by the G-1-P/G7-PTHF feed ratio = 200 and 300. Because this supramolecular polymer, which is composed of bio-related component, i.e., amylose, is novel and has a possibility of new biobased hybrid material, it has potential for practical applications as a new type of amylose-based material in the future.
This work was financially supported by a Grant-in-Aid for Scientific Research from Ministry of Education, Culture, Sports, Science, and Technology, Japan (No. 24350062) and a research grant from The Mazda Foundation. The authors thank Ezaki Glico Co., Ltd., Osaka, Japan for the donation of thermostable phosphorylase.