2016 Volume 63 Issue 3 Pages 51-59
2016 Volume 63 Issue 3 Pages 51-59
Abstract: In this study, a β-glucosidase (PaBG1b) with high specific activity was purified from gut extracts of the wood-feeding cockroach Panesthia angustipennis spadica using Superdex 75 gel filtration chromatography and High-Trap phenyl hydrophobic chromatography. The protein was purified 14-fold to a single band identified by sodium dodecyl sulfate polyacrylamide gel electrophoresis, with an apparent molecular mass of 56.7 kDa. The specific activity of the purified enzyme was 708 μmol/min/mg protein using cellobiose as substrate. To the best of our knowledge, this is the highest specific activity reported among β-glucosidases to date. The purified PaBG1b showed optimal activity at pH 5.0 and retained more than 65 % of the activity between pH 4.0 and 6.5. The activity was stable up to 50 °C for 30 min. Kinetic studies on cellobiose revealed that the Km was 5.3 mM, and the Vmax was 1,020 μmol/min/mg. The internal amino acid sequence of PaBG1b was analyzed, and two continuous sequences (a total of 39 amino acids) of the C-terminal region were elucidated. Based on these amino acid sequences, a full-length cDNA (1,552 bp) encoding 502 amino acids was isolated. The encoded protein showed high similarity to β-glucosidases from glycoside hydrolase family 1. Thus, the current study demonstrated the potential of PaBG1b for application in enzymatic biomass-conversion as a donor gene for heterologous recombination of cellulase-producing agents (fungi or bacteria) or an additive enzyme for cellulase products based on the high-performance of PaBG1b as a digestive enzyme in cockroaches.
SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; PCR, polymerase chain reaction; GH, glycoside hydrolase.
β-Glucosidase (EC 3.2.1.21) is one of key components in cellulolytic systems of all forms of life. In general, a complete cellulolytic system consists of cellobiohydrolases (EC 3.2.1.91 and EC 3.2.1.176), which hydrolyze cellulose chains from the nonreducing or reducing terminus and directly act on crystalline regions of cellulose; endoglucanases (EC 3.2.1.4), which hydrolyze cellulose chains randomly; and β-glucosidases, which eliminate cellobiose accumulated after the activities of the other components of the system, resulting in production of glucose.1) Cellobiose accumulation, which causes inhibition of cellobiohydrolase activities, is a major limiting factor,2) and limitations of cellulose hydrolysis activity may account for the low production of β-glucosidase in hyperactive enzyme secretory strains of Trichoderma.3) Accordingly, β-glucosidases are key enzymes that greatly affect the overall performance of cellulolytic systems, and highly active β-glucosidases are needed to improve the performance of cellulolytic organisms, such as T. reesei.2)3) Moreover, within the typical environment used in biomass conversion, where dramatic accumulation of cellobiose can occur, higher specific activities are required for β-glucosidases, and these enzymes must also exhibit increased thermostability and reduced inhibitory effects against glucose.4)5)
Insects are an attractive resource for cellulolytic enzymes, and some species are well adapted to lignocellulose digestion.6) The intestinal lumen of wood-feeding insects is recognized as an effective natural biomass conversion system. For example, termites harbor a dual digestive system containing endogenous enzymes and symbiotic protozoan fauna,7) and the digestive system of wood-feeding cockroaches depends on endogenous enzymes (the protozoa-inhabiting cockroach Cryptocercus punctulatus is the only known exception), another typical cellulolytic system that represents the basis for the evolution of termites (now part of the cockroach lineage in the order Blattodea8)9)).6) The luminal space, particularly that of the midgut, is filled with finely powdered wood particles that are crushed and ingested by host insects to particles as small as 10 μm in length in the case of the termite Coptotermes formosanus; this space also has extremely high concentrations of endoglucanase and β-glucosidase to facilitate glucose production.6)10) Thus, the cellulolytic system of xylophagous insects is considered an evolutionary producer of high-performance cellulolytic enzymes.
Here, we report the purification and cloning of a β-glucosidase (PaBG1b) from the gut of the wood-feeding cockroach Panesthia angustipennis spadica and its remarkable efficiency in cellulose hydrolysis.
Preparation of crude-enzyme extracts. Panesthia angustipennis spadica (Fig. 1; midsized and above nymphs, sex nondiscriminated) were collected in the northern part of Tsukuba city from decayed wood logs in a forest. The collected cockroaches were anesthetized in flaked ice and dissected. The midguts were collected and stored at −28 °C until use. The midguts were homogenized in sodium acetate buffer (0.1 M, pH 5.0 or 5.5 with or without 150 mM NaCl) containing 1 × Complete Mini protease inhibitor cocktail (Roche Ltd., Basel, Switzerland) using an ultrasonic homogenizer (POLYTRON®, Kinematica AG, Luzern, Switzerland). The homogenate was centrifuged (5,000–15,500 × G for 5–10 min), and the liquid (upper) layer was recovered. The recovered fraction filtered through a membrane filter (pore size, 0.22 μm; Milex GV; Sigma-Aldrich Corporation, St. Louis, USA) was referred to as the crude extract. Proteins in the crude extract were precipitated with three volumes of cold acetone to reduce volume and remove fat. Precipitated samples were dissolved into an appropriate volume of sodium acetate buffer and applied to liquid chromatography.

A male adult (middle) lost its right antenna. Nymphs (right) exhibited medium sizes. First instar nymphs are born oviparously at around 5 mm in body length, and full-grown adults are around 30 mm in length.
Liquid chromatography. Purification of β-glucosidase was performed with a combination of hydrophobic-interaction chromatography, anion-exchange chromatography, and gel filtration.
Hydrophobic-interaction chromatography was performed using a HiPrep Phenyl FF (high sub, 1 mL) column (GE Healthcare, Little Chalfont, England) with two different methods. First, the column was equilibrated with sodium acetate buffer (100 mM, pH 5.5) with 1.2 M ammonium sulfate. Samples were applied with equilibration buffer, and protein elution was started by reducing the concentration of ammonium sulfate to 0.2 M, with an addition linear decreasing gradient from 0.2 to 0 M (buffer condition 1). In another method, the column was equilibrated with sodium acetate buffer (100 mM, pH 5.0) containing 150 mM NaCl. Samples were applied with sodium acetate buffer, and proteins were eluted with linear decreasing gradient of NaCl (150 to 0 mM, 30 mL) in sodium acetate buffer (buffer condition 2).
Anion-exchange chromatography was performed using a Mono Q 5/50 GL column (GE Healthcare), and cation-exchange chromatography was performed using a Mono S 5/50 GL column (GE Healthcare). Both columns were equilibrated with sodium acetate buffer without NaCl, and samples were applied under the same buffer conditions. Proteins were eluted with a linear gradient of NaCl (0 to 150 mM, 30 mL).
Gel filtration was performed using a Hi-Load Superdex 75 (16/600) prepacked column (GE Healthcare) equilibrated and eluted with 0.1 M sodium acetate buffer with NaCl (150 mM).
For preparation of samples for kinetic studies, the crude extract was subjected to gel-filtration (Hi-Load Superdex 75) and hydrophobic-interaction chromatography (HiPrep Phenyl FF) under buffer condition 1 and gel-filtration (hydrophobic-interaction chromatography) sequentially after acetone precipitation. For internal amino acid sequencing, the crude extract was directly applied to the HiPrep Phenyl FF column under buffer condition 2, followed by anion-exchange chromatography (Mono-Q), and finally gel filtration.
Concentration of samples. Recovered fractions from hydrophobic chromatography were concentrated using a centrifuge ultrafiltration tube with polyvinylidene difluoride membranes (Macrosep Advanced Centrifugal Device, Pall Corporation, Ann Arbor, USA) or using a polysulfone ultramembrane (cut-off size, 10,000 Da, 76 mm; Advantec Toyo Kaisha, Ltd., Tokyo, Japan) attached to a stirred ultrafiltration cell (Amicon #8400; Merck Millipore, Darmstadt, Deutschland) pressurized with nitrogen gas.
Measurement of enzymatic activity. β-Glucosidase activity was measured as glucose production per min from cellobiose by the mutarotase-GOD method11)12)13) using Glucose C2 (Wako Pure Chemical Industries, Ltd., Osaka, Japan). For chromatographic activity curve drawing, samples (10 μL) were added to 40 μL of 1 % (w/v) cellobiose solution in 0.1 M sodium acetate buffer (pH 5.5) and then incubated at 37 °C for 5 min. After incubation, 200 μL of Glucose CII reagent was added immediately (the β-glucosidase reaction was minimized by dilution), and samples were then left at room temperature for 5 min. The absorbance (505/595 nm) of 150 μL of the reaction mixture was measured using a microplate reader (model 680, Bio-Rad Laboratories Inc., Hercules, USA). The obtained values (505–595 nm) were used for drawing an elution profile of β-glucosidase activity.
For quantitative measurement of the activity of the purified enzyme, a standard glucose solution (0.5 mM) and serial dilutions were measured simultaneously using the same method. Diluted sample enzyme solutions (25 μL) were added to 100 μL of 1 % cellobiose solution (pH 5.5, in 100 mM sodium acetate buffer) and incubated at 37 °C for 5 min. The reaction was stopped by heating (95 °C, 10 min), and 600 μL Glucose C2 reagent was added. Samples were then incubated for 4 min at 37 °C, and absorbance at 505 nm was measured using a 10-mm crystal cuvette with a spectrometer. Serial dilutions of cellobiose (0.01–2.00 %, w/v [0.29–58.4 mM]) were prepared for kinetic studies. For measurement of optimum temperature, the purified enzyme was reacted at various temperatures (10–70 °C). For measurement of thermal stability, the purified enzyme was pre-incubated at various temperatures (10–70 °C), and activity was then measured quantitatively. For measurement of pH/activity profiles, McIlvane’s broad range buffer14) was used for cellobiose solution instead of sodium acetate buffer, and samples were diluted appropriately (50×) with MilliQ water to avoid influence of sample buffer. One unit (U) of β-glucosidase activity was defined as 2 μmol/min of glucose production from cellobiose because one molecule of cellobiose was converted into two molecules of glucose through β-glucosidase activity.
Measurement of protein concentrations. Protein concentrations of samples were measured by the Bradford method15) using Quick Start Bradford Protein Assays with a Quick Start Bovine Serum Albumin (BSA) Standard Set (Bio-Rad) according to the manufacturer’s instructions.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). SDS-PAGE16) was performed with protein markers (Precision Plus Protein Standards; Bio-Rad) using Mini-Protean TGX Gels (Bio-Rad) according to the manufacturer’s instructions. Proteins were stained with Oriole Fluorescent Gel Stain (Bio-Rad) and observed and photographed on a ultraviolet light box (254 nm) or were transblotted onto polyvinylidene fluoride membranes (Trans-Blot SD, Bio-Rad) and stained with Coomassie Brilliant Blue. Molecular weight markers run with the samples were recombinant nonstructural protein markers (10–250 kDa, XL-Ladder Broad, APRO Science K.K., Tokushima, Japan) or Precision Plus Protein Standards (Bio-Rad).
Internal amino acid sequencing. Samples were digested using lysine-specific serine protease (Lysyl Endopeptidase, Wako Pure Chemical Industries). Protein fragments were separated by reversed-phase high-performance liquid chromatography (XBridge BEH300, C18, 3.5 μm; Waters Corporation, Milford, USA), and N-terminal amino acid sequencing was performed using a Procise cLC Sequencing System (Model 491cLC; Applied Biosystems, Foster City, USA).
First-strand cDNA synthesis and polymerase chain reaction (PCR). mRNA was extracted from the mixture of the salivary glands and front terminal tissue of the midgut using QuickPrep micro mRNA extraction kit (GE Healthcare). First-strand cDNA was synthesized using a SMART II cDNA RACE amplification kit (Clontech Laboratories Inc., Mountain View, USA) with recombinant moloney murine leukemia virus reverse transcriptase modified to lose RNaseH activity (ReverTra ACE, Toyobo Co., Ltd., Osaka, Japan). Based on the internal amino acid sequences, degenerate primers (PaBG_degenerate_R01 and PaBG_degenerate_F01; Table 2) were designed, and the corresponding cDNA fragments were amplified using KOD Fx DNA polymerase (Toyobo). The PCR protocol included 30 cycles at 98 °C for 10 s, 47 °C for 30 s, and 68 °C for 30 s after initial denaturation at 94 °C for 2 min. Gene-specific primers (Table 2) were designed based on the obtained cDNA fragment, and 5′- and 3′-rapid amplification of cDNA ends (RACE) were performed using a SMART II cDNA RACE amplification kit (Clontech) according to the manufacturer’s instructions. The sequence was determined using a 3730XL Genetic Analyzer with a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems).
Sequence deposition. The obtained sequence was deposited in GenBank/DDBJ/EMBL database under the accession number LC125463.
A total of 3,540 U of β-glucosidase activity was found in the crude extract from the midguts of 50 cockroach nymphs using cellobiose as substrate. Proteins in the crude extract were precipitated with three volumes of cold acetone and dissolved in acetate buffer (pH 5.5). Acetone precipitation and dissolving improved specific activity by 1.6-fold compared with that of the crude extract, and the dissolved sample was applied on the gel-filtration column (Table 1 and Fig. 2). The recovered fraction from the gel-filtration was further purified by chromatography on a HiTrap Phenyl column with 1.2 M ammonium sulfate and eluted with lower ammonium sulfate concentrations (< 0.2 M; Fig. 3). The recovered fractions (350–700 mL) were concentrated by ultrafiltration to 40 mL. The obtained protein was observed as a single band by SDS-PAGE (Fig. 4), and the molecular mass was estimated to be 56.7 kDa by comparing its mobility with standard proteins. β-Glucosidase of Panesthia angustipennis was purified 14 fold, and the specific activity was 708 U/mg. The recovered protein was concentrated, and the buffer was replaced with 0.1 M sodium acetate (pH 5.5) for kinetic studies using the ultrafiltration device.
| Units (μmol glucose/min) |
Protein (mg) |
Specific activities (U/mg protein) |
Purification folds | Recovery (%) |
|
|---|---|---|---|---|---|
| Crude extract | 3540 | 69 | 130 | 1 | 100 |
| Acetone precipitation | 2790 | 34 | 166 | 1.6 | 79 |
| Superdex-75 | 1640 | 19 | 175 | 1.7 | 46 |
| HiPrep Phenyl FF | 840 | 1.2 | 708 | 14 | 24 |

Activities (lateral bars) are described as percentages of the peak value. The protein curve (broken line) indicates the relative absorbance (280 nm). Recovery is indicated by the horizontal bar.

The inset shows SDS-PAGE of the eluted fractions (A-P) negatively stained with Oriole. The activity curve is presented as relative values (%) based on the highest activity in the fractions.

S, Purified β-glucosidase; M, Precision Plus Protein Standards (Bio-Rad). The numbered molecular weight markers were used for estimation of the molecular weights of proteins in the sample.
The purified enzyme showed highest activity at pH 5.0 and maintained at least 65 % activity between pH 4.0 and 6.5. The enzymatic activity increased proportionally between 10 and 60 °C, then declined rapidly, with complete loss of activity at 70 °C (optimum activity was observed at 60 °C). The enzyme showed thermal stability up to 50° C for 30 min of pre-incubation.
Lineweaver-Burk plot17) of the purified enzyme (Fig. 5) and the molecular weight on SDS-PAGE yielded Km, Vmax, kcat, and kcat/Km values of 5.3 mM, 1,020 U/mg, 967 /s, and 184 mM/s, respectively.

The concentration of the purified enzyme used for these plots was 0.012 mg/mL, and the regression line was given as: 1 / (β-glucosidase activity [U/mL]) = −0.21 × 1 / (cellobiose concentration [mM]) + 0.04.
N-terminal amino-acid sequencing of the purified β-glucosidase resulted in ambiguous sequences of several unrelated peptides. To address whether this was due to N-terminal blocking of the target enzyme or contamination by other proteins, the crude extract of the midguts from nine mid-size cockroach nymphs was subjected to a combination of hydrophobic interaction chromatography (HighPrep Phenyl), anion-exchange chromatography (Mono-Q), concentration with the centrifuge ultrafiltration tubes, and gel-filtration chromatography (Superdex 75). The purified enzyme showed only 10 % higher specific activity (790 U/mg), and the identity of the purified β-glucosidase was confirmed by the motility of the purified protein on SDS-PAGE and by the identical elution profile on gel-filtration, suggesting that there was little contamination by other proteins. The purified sample was subjected to internal amino acid sequencing, which successfully resulted in three fragmental amino acid sequences [(K)FGLYQVDFEDPTRPRIMK, (K)ESARPFQQIIATRQIPEAYR, and DHGYYLTK]. The second sequence was estimated to be joined as (K)FGLYQVDFEDPTRPRIMKESARPFQQIIATRQIPEAYR based on alignment corresponding to the C-terminal amino acid sequence of a β-glucosidase from the termite Neotermes koshunensis (NkBG, GenBank: AB073638.2, Protein ID: BAB91145.118); Fig. 6). Reverse-transcription PCR with degenerate primers that were designed based on alignment of these sequences resulted in a cDNA fragment (0.1 kbp) (Table 2), which was cloned into a conventional TA-cloning vector and sequenced. From the obtained sequence, gene-specific primers were designed (Table 2). A complete cDNA sequence of 1,552 bp in length (PaBG1b; LC125463) was obtained by 5′- and 3′-RACE and encoded a putative protein of 502 amino acids showing high homology to β-glucosidases from glycoside hydrolase family 1 (GH1)19); thus, we assumed that the purified protein belonged to this same family. The first 21 amino acids at the N-terminus were highly hydrophobic and presumed to be a signal peptide based on the SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP/).20)21) The calculated molecular weight of the deduced peptide sequence (without the signal peptide) was 55.3 kDa, which is slightly smaller than the apparent molecular weight of the purified β-glucosidase. This difference could be explained by possible glycosylation at the two potential N-glycosylation sites at Asn265 and Asn416. PaBG1b showed 99.0 and 99.3 % identities in nucleotide and amino acid sequences, respectively, to PaBGI (BAO85050: 303 amino acids), a partial fragment of a putative β-glucosidase previously reported from the same species.22)
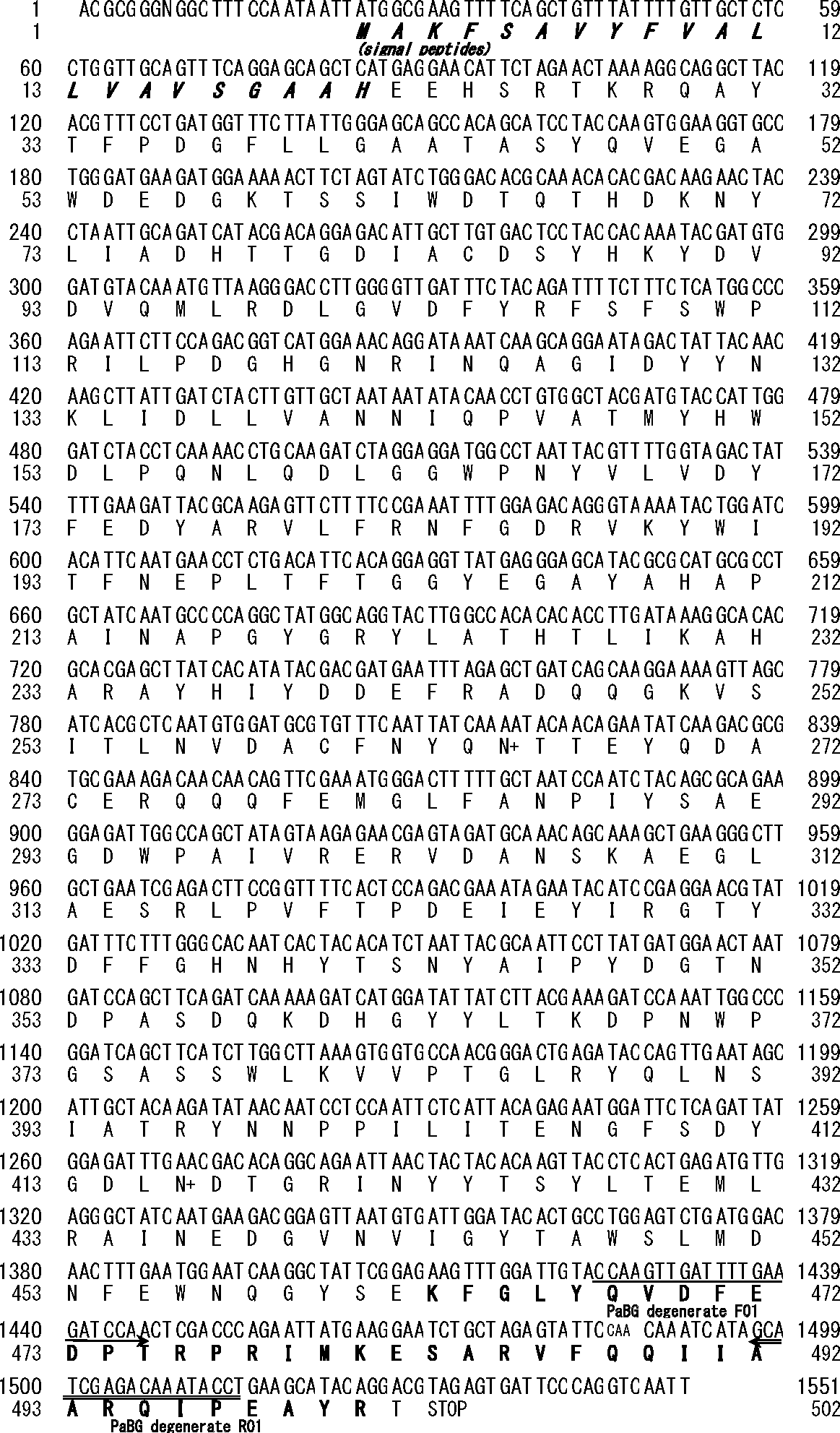
The bold characters in the amino acid sequence indicate consentaneity with the internal amino acid sequences. Double and single underlines with arrow heads indicate positions of the degenerate primers. Potential N-glycosylation sites are designated by “+” on the right side of the corresponding amino acids.
| Name | Position in PaBG1b (LC125463, 1551 bp) |
Side | Sequence (5′ to 3′) |
|---|---|---|---|
| PaBG_degenerate_F01 | 1425-1447 | Forward | GCACTNGAYTTCGARGACCCAAC |
| PaBG_degenerate_R01 | 1519-1497 | Reverse | CGCTCWGGWATCTGNCTTGTNGC |
| PaBG_GSP_F01 | 1440-1467 | Forward | GAcCCAACTCGACCCAGAATTATGAAGG (the “c” at the third nucleotide is a “T” in the real sequence) |
| PaBG_GSP_F02 | 1448-1474 | Forward | TCGACCCAGAATTATGAAGGAATCTGC |
| PaBG_GSP_F03 | 1466-1493 | Forward | GGAATCTGCTAGAGTATTCCAACAAATC |
| PaBG_GSP_R01 | 1467-1440 | Reverse | CCTTCATAATTCTGGGTCGAGTTGGgTC (the “g” at the 26th nucleotide is an “A” in PaBG1b) |
| PaBG_GSP_R02 | 1474-1448 | Reverse | GCAGATTCCTTCATAATTCTGGGTCGA |
| PaBG_GSP_R03 | 1493-1466 | Reverse | GATTTGTTGGAATACTCTAGCAGATTCC |
| PaBG_GSP_R04 | 1535-1508 | Reverse | ACTCTACGTCCTGTATGCTTCAGGTATT |
Although most cockroaches are omnivorous, wood-feeding lineages have evolved multiple times in the evolution of cockroaches.23) Cockroaches of the genus Panesthia are found in rotting wood and reported to have the efficient cellulolytic capability.24)25) To the best of our knowledge, the purified β-glucosidase PaBG1b from Panesthia angustipennis spadica in the present study showed the highest activity on cellobiose among β-glucosidases reported to date.
Hydrophobic chromatography is an effective method for purification of cellulolytic enzymes. The present study showed that our sample exhibited a broad elution profile on HiTrap Phenyl, in contrast to our previous study wherein we observed sharp elution of endoglucanse (EcEG) from the gut extract of the stick insect Eurycantha calcarata.26) In a previous study, the elution buffer was 20 mM Tris-HCl, and the salt gradient was formed with ammonium acetate. Acetate buffers (in many cases 0.1 M, pH 5.5) have historically been used for cellulolytic assays and purification of termites and wood-feeding cockroaches.10)24)27)28)29)30)31)32)33)34)35)36)37) In the current study, we followed buffer usage protocols described in previous studies. Thus, we used sodium acetate buffer at a relatively high concentration (0.1 M), which may explain the broad elution patterns on hydrophobic chromatography. After the decrease in ammonium acetate concentration to zero, there was a relatively high concentration of salt (sodium acetate, 0.1 M) remaining in the buffer; this may have prevented sharp elution of the target protein. This choice necessitated the additional step of concentration of large volumes of recovered fractions using ultramembranes; however, the specific activity of the concentrated protein was increased to over 700 U/mg. Owing to the difficult sample handling procedures required, we do not recommend using sodium acetate buffer (0.1 M) for purification of insect cellulolytic enzymes with hydrophobic chromatography; however, we believe that the current results demonstrate the possible outcomes of this method.
To the best of our knowledge, prior to our study, the β-glucosidase reported to have the highest specific activity on cellobiose was derived from the thermophilic fungus Thermoascus aurantiacus (Table 3).38) However, the Vmax of this previously characterized β-glucosidase (TaBG3) on cellobiose was estimated to be 783.7 U/mg; thus, Vmax of TaBG3 was equivalent to 76.8 % of those of PaBG1b, as reported in the present study. Although the Km of PaBG1b was not lower than average among β-glucosidases from various organisms (Table 3), the kcat/Km was relatively high (Table 3). Thus, PaBG1b may be the most effective enzyme in the presence of abundant amounts of substrate.
| Species | Vmax (U/mg) |
Km (mM) |
kcat/Km (/mM/s) | Optimal temperature (°C) |
GH family | Reference |
|---|---|---|---|---|---|---|
| Invertebrates | ||||||
| P. angustipennis | 1020 | 5.3 | 184 | 60 | 1 | This study |
| Reticulitermes flavipes (RfBGGluc-1) | 638 | 1.44 | 414 | n.d. | 1 | 43) |
| Neotermes koshunensis (NkBG)* | 110 | 3.8 | 28.9 | 50 | 1 | 40) |
| Coptotermes formosanus | 462.6 | 2.3 | 188 | 49 | 1 | 44)45) |
| Plants | ||||||
| Oriza sativa (Os3BGlu6) | 0.15 | 15.3 | 0.0085 | 55 | 1 | 46) |
| Fungi | ||||||
| Debaryomyces vanrijiae | 84.3 | 57.9 | 2.43 | 40 | Unknown | 47) |
| Aspergillus orizae | 353 | 7 | 36.1 | 50 | Unknown | 48) |
| A.niger | 31.1 | 1.11 | 60.6 | n.d. | 3 | 49) |
| Candida peltata | 66 | 8.5 | 0.1 | 50 | 50) | |
| Humicola insolens (BglHi1) | 86 | 0.51 | 151.8 | n.d. | 1 | 51)52) |
| Humicola insolens (BglHi2) | 46.5 | 0.24 | 1304.9 | 65 | 3 | 52) |
| Thermoascus aurantiacus (TaBG3) | 783.7 | 0.67 | 1579.1 | 65 | 3 | 38) |
| Bacteria | ||||||
| Thermoanaerobacterium thermosaccharolyticum | 120 | 7.9 | 13.3 | 70 | 1 | 53 |
| Uncultured bacterium | 15.5 | 20.4 | 0.65 | 40 | 1 | 54 |
| Unidentified organism (putatively uncultured bacterium) from Kusaya gravy (Ks5A7) | 155 | 0.36 | 197 | 50 | 1 | 55 |
| Archaea | ||||||
| Sulfolobus solfataricus (SSO3039) | 0.4 | 2.1 | 0.9 | 70 | 116 | 56 |
This table lists only enzymes for which properties have been investigated with cellobiose as a substrate. Notes: 1 U = 2 μmol glucose production/min. It is probable that the Vmax values reported in some of these studies are half of the values listed above because 1 U was defined as 1 μmol production/min. However, we have presented the results here as reported, regardless of the lack of a clear definition of enzymatic activity. *Since this study defined 1 U as 1 μmol glucose production/min, the Vmax is shown as half of the reported value, which was then used to estimate the kcat.
The sequence PaBG1b showed highest homology (E-value 0.0) to a β-glucosidase from the American omnivorous cockroach Periplaneta americana (AIA09348: 505 amino acids) and a β-glucosidase of the termite N. koshunensis (NkBG, BAB91145: 498 amino acids).18) High homology was also observed for the putative β-glucosidase fragment (PaBGI; the enzymatic activity has not been investigated) from Panesthia angustipennis spadica collected from different locations,22) all of which belong to GH1. All crucial amino acid residues putatively involved in catalysis, such as the proton donor Glu196 and the nucleophile Glu406, as well as those involved in substrate binding, such as Asn416, were conserved in PbBG1b (Fig. 6), confirming that this protein encoded a functional β-glucosidase. Because the protein contained a hydrophobic signal peptide (Fig. 6), PaBG1b may be a secretory enzyme involved in digestion. Marked differences in sequences were not observed when compared with other digestive β-glucosidases involved in cellobiose hydrolysis, which have been isolated from termites (results not shown).
Although some β-glucosidases are known to be involved in social communication, a previous study indicated that these β-glucosidases are grouped into the BGI clade, which primarily consists of digestive β-glucosidases,22) supporting that PaBG1b also participates in cellulose digestion in Panesthia angustipennis. Because insect EGs are not very active against crystalline cellulose and Panesthia angustipennis spadica does not harbor the symbiotic protists that help digestion of cellulose,6) cockroaches may have developed efficient β-glucosidases for production of sufficient levels of glucose from cello-oligomers released by the actions of EGs to survive on rotting wood. Digestive enzymes of insects have not been adapted to high temperatures in general because insects are poikilothermic, and their digestion occurs at environmental temperatures unlike fermenting fungi, such as Trichoderma spp. Thus, low thermal stability and different pH optima may be disadvantages of insect-derived enzymes compared with commercial fungal β-glucosidases. However, recent advancements in evolutionary engineering may provide opportunities for improvement and recombinant production of PaBG1b, as shown in the case of improved thermal stability of the termite endoglucanase by family shuffling of four orthologous cDNAs.39) Such an improvement in PaBG1b may also result in opportunities for recombination with cellulolytic fungus genes. In addition, previous studies have reported efficient production of termite β-glucosidase using microorganisms such as Escherichia coli, Pichia pastoris, and Aspergillus oryzae.40)41)42) A similar strategy for heterologous expression of recombinant PbBG1b may significantly contribute to efficient saccharification of cellulosic biomass in industrial applications.
This study was supported by the New Energy and Industrial Technology Development Organization (Project ID: 08150160), Bio-oriented Technology Research Advancement Institution (Project ID: 07807165), and KAKENHI from JSPS (No. 26292177).