2017 Volume 64 Issue 2 Pages 27-32
2017 Volume 64 Issue 2 Pages 27-32
Lactulose, a disaccharide widely used in pharmaceuticals and functional foods, is produced by lactose isomerization. Lactose and lactulose have an aldose–ketose relationship. Less than 25 % conversion of lactose into lactulose is achieved using the Lobry de Bruyn–Alberda van Ekenstein transformation with heating, whereas the conversion is increased to 80 % by the addition of an approximately equimolar concentration of the organogermanium compound 3-(trihydroxygermyl)propanoic acid (THGP) to the reaction mixture. To further understand this phenomenon, in this study, we analyzed the affinity between THGP and sugar isomers using 1H nuclear magnetic resonance spectroscopy. For the dimethyl derivative of THGP with lactose and lactulose, the complex formation ratios at 0.1 M (1:1 mixing ratio) were 14 and 59 %, respectively, with complex formation constants of 1.8 and 43 M–1, respectively. The complex formation capacity was approximately 24-fold higher for lactulose than for lactose. Moreover, THGP is considered to protect lactulose from alkaline degradation, resulting in high production yield of lactulose. Therefore, we concluded that high affinity for the isomerization product may promote isomerization and that promotion of sugar isomerization using an organogermanium compound is an effective method for converting lactose to lactulose.
DM-THGP, 3,3-dimethyl-3-(trihydroxygermyl)propanoic acid; Ge-132, poly-trans-[(2-carboxyethyl)germasesquioxane]; HPLC, high-performance liquid chromatography; Ks, complex formation constant; NMR, nuclear magnetic resonance; THGP, 3-(trihydroxygermyl)propanoic acid.
Recently, alkaline isomerization from glucose to fructose has received much attention as a process for producing a sweetener containing a rare sugar,1) fuel from biomass, and chemical intermediates.2)3) We previously described the effective promotion of the conversion of D-glucose into D-fructose using organogermanium compounds with a 3-(trihydroxygermyl)propanoic acid (THGP) structure, in both enzymatic and alkaline isomerization reactions.4)
Lactose (4-O-β-D-galactopyranosyl-D-glucose) is a disaccharide composed of galactose and glucose, which is contained in cow's milk (approximately 5 %). Also, approximately the same amount of lactose (5 %) is contained in whey, a byproduct of cheese production. In contrast, lactulose (4-O-β-D-galactopyranosyl-D-fructose) is a disaccharide composed of galactose and fructose that can be produced by lactose isomerization.5) Lactulose, which has been used for the treatment of hepatic encephalopathy6) and constipation,7) is on the World Health Organization Model List of Essential Medicines,8) a list of the most important medications needed in a basic health system. Examples of its in vivo actions include growth of bifid bacteria, suppression of ammonia absorption, and increase in intestinal water content.9)
Currently, an efficient isomerizing enzyme for lactose is not available; therefore, alkaline isomerization is the sole method for lactulose production. In alkaline isomerization, however, the conversion from lactose to lactulose is very low (21.4 %).10) Therefore, in the present study, we applied the method4) of alkaline isomerization in the presence of THGP to increase the conversion of lactose to lactulose. THGP can be obtained by hydrolyzing the organogermanium compound poly-trans-[(2-carboxyethyl)germasesquioxane] (Ge-132) in aqueous solution. Ge-132, which is a polymer with the rational formula [(GeCH2CH2COOH)2O3]n, has been shown to be safe in a variety of toxicity studies,11)12)13)14)15)16)17)18)19) and has recently been used as a material for supplements and cosmetics.
This study investigated the following points: (1) evaluation of the molar ratio of added THGP and the relationship between lactulose yield, ratio of remaining lactose, galactose yield, and pH value; (2) evaluation of the affinity between THGP and sugar isomers and determination of the complex formation constants (Ks) using the 1H nuclear magnetic resonance (NMR) reporter molecule 3,3-dimethyl-3-(trihydroxygermyl)propanoic acid (DM-THGP); and (3) consideration of the mechanism by which THGP promotes alkaline isomerization of lactose to lactulose.
Reagents. The structures of organogermanium compound THGP and 1H-NMR reporter molecule DM-THGP used in this study are shown in Fig. 1. These organogermanium compounds were synthesized at Asai Germanium Research Institute Co., Ltd. (Kanagawa, Japan). Lactose monohydrate (guaranteed reagent grade) and sodium hydroxide (guaranteed reagent grade) were purchased from Kanto Chemical Co., Ltd. (Tokyo, Japan). Lactulose (approximately 98 %) was purchased from Sigma-Aldrich Co. LLC (Tokyo, Japan). D-Galactose (guaranteed reagent grade) and deuterium oxide (99.9 % deuteration ratio) were purchased from Merck KGaA (Darmstadt, Germany). Distilled water was prepared by using Advantec fully automatic distilled water manufacturing equipment (GSR-200; Advantec Toyo Kaisha, Ltd., Tokyo, Japan).
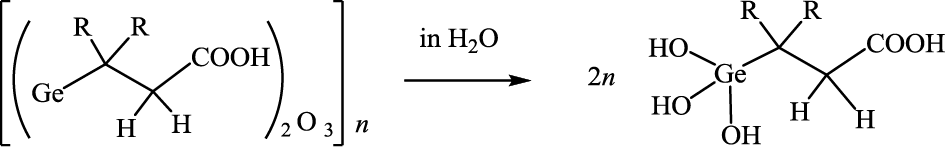
3-(trihydroxygermyl)propanoic acid (THGP) and 3,3-dimethyl-3-(trihydroxygermyl)propanoic acid (DM-THGP) can be obtained by hydrolyzing poly-trans-[(2-carboxyethyl)germasesquioxane] (Ge-132) and poly-[(1,1-dimethyl-2-carboxyethyl)germasesquioxane] in water, respectively.
THGP: R=H; DM-THGP: R=CH3.
Alkaline isomerization reaction. To prepare 4 M THGP solution (pH 7), Ge-132 was dissolved in distilled water and the pH was adjusted using 10 M sodium hydroxide. The reaction mixtures were prepared by adding 1.10−5.28 mL of 4 M THGP (pH 7) to 0.0176 mol lactose in 13–15 mL of distilled water. The reaction mixtures were adjusted to pH 12 using 0.83−1.84 mL of 10 M sodium hydroxide. The lactose concentration in the reaction mixture was kept constant at 0.88 M. The THGP concentrations in the reaction mixture were 0.22, 0.44, 0.66, 0.70, 0.79, 0.88, 0.97, and 1.1 M, with lactose-to-THGP molar ratios of 1:0.25, 1:0.50, 1:0.75, 1:0.80, 1:0.90, 1:1.0, 1:1.1, and 1:1.2, respectively. A control solution (molar ratio of 1:0), which is the normal reaction solution, was prepared with distilled water rather than THGP. All preparation steps were performed under ice-cold conditions. Reaction mixtures (1 mL each) were incubated at 60 °C with shaking (120 rpm) for 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, or 60 min and then quickly placed in ice water.
Analysis of lactose, lactulose, and galactose using high-performance liquid chromatography (HPLC). The reaction mixtures were diluted 60–180-fold with distilled water and then centrifuged for 3 min at 13,500 × G at 1 °C. The lactose, lactulose, and galactose contents in the supernatants were determined by HPLC using a Shimadzu LC-10A system with a LC-10AD pump (Shimadzu Corporation, Kyoto, Japan), RID-6A refractive index detector (Shimadzu Corporation), and C-R7A integrator (Shimadzu Corporation) under the following conditions: column, Polyspher CHPB column (7.9 φ × 300 mm; Merck KGaA, guard column, Polyspher CHPB column (4 φ × 50 mm; Merck KGaA); column temperature, 80 °C; mobile phase, distilled water; and flow rate, 0.4 mL/min. The sample volume applied was 10 μL.
NMR measurements. NMR spectra were recorded at 20 ± 0.5 °C using a Varian Gemini 300 spectrometer (Agilent Technologies, Santa Clara, USA) equipped with a C/H dual probe (diameter: 5 mm). Chemical shifts were expressed in ppm relative to the H resonance of HOD in the solvent, which was offset by δ = 4.80 ppm.
Comparison of the affinity of DM-THGP for lactose and lactulose. To elucidate the mechanism through which the THGP structure promotes alkaline isomerization, the Ks values for lactose and lactulose with DM-THGP were measured, as described previously.4) First, the complex formation ratios for lactose and lactulose in the presence of DM-THGP were measured using 1H-NMR spectroscopy under the following conditions: constant sugar concentration of 0.10 M, DM-THGP concentrations of 0.0250, 0.0375, 0.0500, 0.0750, 0.100, and 0.125 M, and neutral pH (near 7.0). Subsequently, the 1H-NMR integral values of the sugar (0.10 M constant concentration) were used to correct the concentration of DM-THGP. The complexation ratios were calculated using the following equation: Cr = Ic/(Ic + If), in which Cr, Ic, and If are the complexation ratio, integral value of the methyl proton signal corresponding to the DM-THGP–sugar complex, and integral value of the methyl proton signal corresponding to DM-THGP, respectively. Then, using the complexation ratios obtained from the above formula, the Ks values of DM-THGP for lactose and lactulose were calculated from the slope of the complex concentration/free sugar concentration ratio plotted against the free DM-THGP concentration.
Effects of organogermanium compound THGP on alkaline isomerization.
Figure 2 shows the results for isomerization from lactose to lactulose under alkaline conditions in the presence of THGP. The lactulose yield was maximized at 24 % under normal reaction conditions (Fig. 2A, 1:0). In contrast, the maximum yield of lactulose increased to 80 % upon addition of THGP to the reaction mixture (Fig. 2A). When the molar ratio of lactose to THGP in the reaction mixture exceeded 0.75, the maximum yield of lactulose was over 70 % (Fig. 2A, 1:0.75–1.2). Moreover, the maximum yield of lactulose was 78–80 % when the molar ratio of lactose to THGP was 0.9 or more (Fig. 2A, 1:0.90–1.2). Even when equimolar or higher amounts of THGP were added, the maximum yield of lactulose did not change.
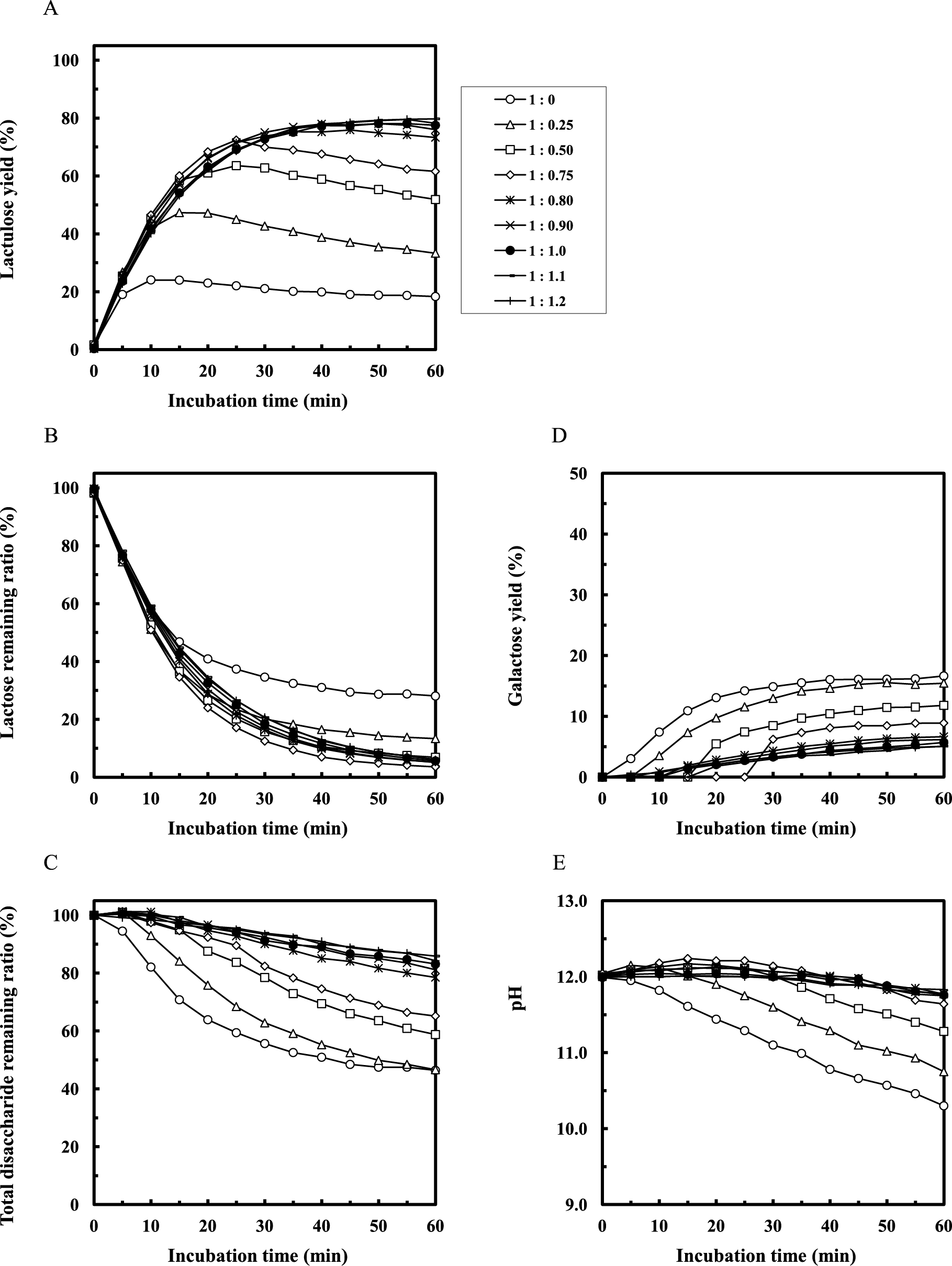
(A) Lactulose yield = lactulose concentration at each reaction time/initial lactose concentration × 100. (B) Lactose remaining ratio = lactose concentration at each reaction time/initial lactose concentration × 100. (C) Total disaccharide remaining ratio = lactose remaining ratio + lactulose yield at each reaction time. (D) Galactose yield = galactose weight concentration at each reaction time/initial lactose weight concentration × 100. (E) pH value. The concentration of each sugar was determined by HPLC.
Figures 2B–E show the ratio of remaining lactose, the ratio of remaining total disaccharides, the yield of galactose, and the pH value, respectively. The total amount of disaccharides decreased under alkaline conditions (Fig. 2C). Under normal reaction conditions for 60 min, the maximum amount of remaining lactose was 28 % (Fig. 2B, 1:0), and the minimum amount of remaining total disaccharides was 46 % (Fig. 2C, 1:0). The maximum yield of galactose derived from alkaline degradation of disaccharides was 17 % under normal reaction conditions (Fig. 2D, 1:0). However, the release of galactose was suppressed by adding THGP (Fig. 2D, 1:0.50−1.2). The pH decreased with progress of the reaction (Fig. 2E), with the most drastic decrease in pH observed under normal reaction conditions (Fig. 2E, 1:0). However, this pH reduction was suppressed by adding THGP (Fig. 2E, 1:0.25–1.2). Finally, addition of THGP to the alkaline isomerization reaction increased the yield of lactulose relative to that under the normal conditions.
Comparison of Ks values for sugar–THGP structures using a 1H-NMR reporter molecule.
The Ks values of THGP with sugar isomers were estimated using DM-THGP as a reporter molecule in 1H-NMR analyses. The proton NMR spectrum of DM-THGP gave only two singlet signals, corresponding to the methyl and methylene groups, which do not exhibit proton coupling (Fig. 3A). However, signals corresponding to the complex between DM-THGP and the sugars were observed near the original signals as multiple shifted signals (Figs. 3B and 3C). The complex formation ratios were calculated from the integral value ratios. For DM-THGP with lactose and lactulose, the complex formation ratios at 0.10 M (1:1 mixing ratio) were 14 and 59 %, respectively. The Ks values of DM-THGP with lactose and lactulose were calculated as described in the Materials and Methods section, using the plots of complex concentration and free sugar concentration versus free DM-THGP concentration shown in Fig. 4. The mean Ks values were Ks(lactose) = 1.8 M–1 and Ks(lactulose) = 43 M–1. Thus, the affinity of DM-THGP for lactulose was approximately 24-fold higher than that for lactose.
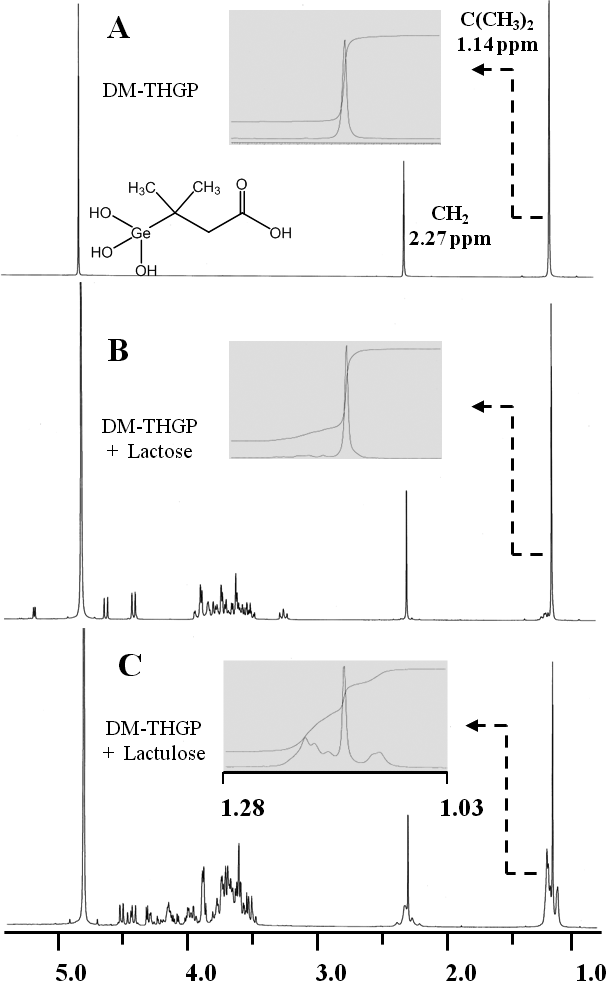
Enlarged spectra of the methyl signals in the 1.03–1.28 ppm region are also shown. (A) DM-THGP; (B) DM-THGP with lactose, 1:1, 0.10 M; (C) DM-THGP with lactulose, 1:1, 0.10 M; 20 °C in D2O at neutral pH.
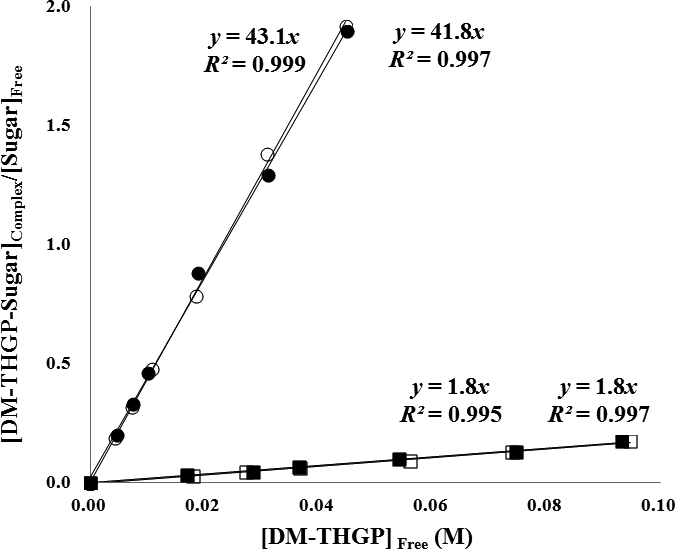
Plots of complex concentration and free sugar concentration versus free DM-THGP concentration at 20 °C in D2O at neutral pH. The experiments were performed twice for both lactose (□ and ■) and lactulose (○ and ●).
As described above, the addition of an equimolar concentration of THGP to the alkaline isomerization reaction increased the lactulose yield by a factor of up to 3.3 with respect to the control. This is considered due to the difference in complex formation capacity of THGP for lactulose and lactose; 24-fold higher for lactulose than for lactose. In addition, the addition of THGP decreased the galactose yield derived from lactulose degradation and suppressed the reduction of the pH during the reaction. Alkaline degradation of 4-O-methyl-D-glucose has been explained using the Nef–Isbell mechanism.20)21) This mechanism can also be applied to lactose and lactulose, as shown in Fig. 5. Galactose is released by alkoxycarbonyl elimination (β-elimination) of the enediol structure (Fig. 5C). As a result, an unsaturated ketone is formed (Fig. 5G), followed by generation of an organic acid, i.e., deoxyaldonic (saccharinic) acid (Fig. 5H). Therefore, the pH of the reaction mixture decreases, and the lactulose yield of the control is reduced by this alkaline degradation. However, as revealed in this study, THGP has a high affinity for lactulose. Therefore, part of the lactulose molecule is negatively charged because of complex formation with the Ge anion of THGP (Fig. 5F). This negative charge protects the lactulose molecule from attack by the alkaline reagent, as negative charges would repel each other. As a result, alkaline degradation which competes with alkaline isomerization, and accompanied pH reduction are suppressed by THGP. These effects of THGP result in production of lactulose in a high yield. Thus, this method may be applicable for efficient alkaline isomerization reactions from various aldose sugars to ketose sugars.
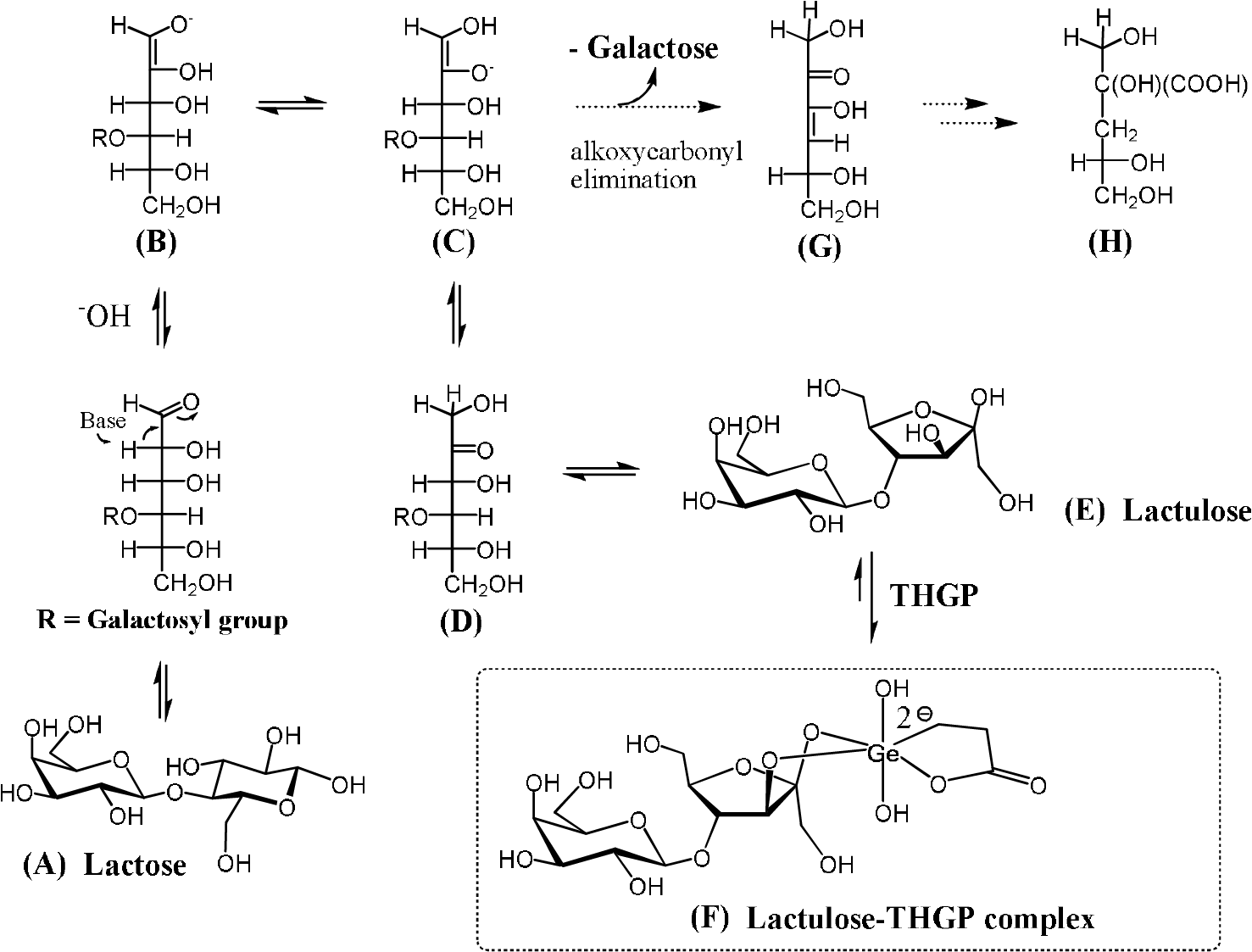
(A), (B), (C), (D), and (E): Alkaline isomerization pathways from lactose to lactulose. (C), (G), and (H): Partial Nef-Isbell mechanism for the alkaline degradation of lactose and lactulose. (F): Stabilization of lactulose by complex formation with THGP.
In summary, the results of the present study indicate that promotion of sugar isomerization upon addition of THGP is an effective method for the conversion of lactose into lactulose. Moreover, we conclude that the high affinity of THGP for the isomerization product may promote isomerization. In view of the reaction mechanism, this method may be useful for the production of ketoses from aldoses by the enrichment of cis-diol-containing structures.22) As this organogermanium compound is relatively expensive, the development of a recycling system for organogermanium compounds using selective capture materials,23) electrodialysis instruments, or materials on which a THGP derivative can be immobilized is necessary for continued advancement in this field.
We are very grateful to Dr. Norihiro Kakimoto and Dr. Keiji Umeda for their helpful advice regarding the study design. We also thank Dr. Mitsuo Akiba for assistance with management of the study. A part of this study was performed as a research project with Yotsuba Milk Products Co., Ltd. at the National Food Research Institute, NARO, Japan, and we thank the researchers who provided assistance with the experiments.