2019 Volume 66 Issue 3 Pages 97-102
2019 Volume 66 Issue 3 Pages 97-102
Ethanol precipitation process for purification of branched dextrin (BD) in Nägeli amylodextrin from waxy rice starch was developed. Temperature and ethanol concentration for precipitation were main parameters affecting the recovery and purity of BD, and the purification condition at 4 °C and 10 % (v/v) ethanol in water was adopted. After four-time precipitation, the BD recovery was 34.6 %, whereas the purity improved from 78.5 % at the initial to 94.5 % at the four-time purified BD (BD4). BD4 mainly showed a chain length distribution between 18 to 35 with a mode length of 25, which shifted after enzymatic debranching with isoamylase to that between 9 and 20 with a mode length of 14. Each purified BD was solubilized in water, and each solution was mixed with methanol-water at 25 °C to a final methanol concentration of 16 M. The flakes of BD precipitated with 16 M methanol exhibited an A-type crystal structure by an X-ray diffraction analysis, and the speed generation of white flakes in 16 M methanol dramatically increased as the purification time increased. The effect of addition of highly branched cyclic dextrin (HBCD) or sodium tetraborate on BD aggregation in 16 M methanol was also investigated, where the former retarded aggregation but the latter had no effect on the velocity. Thus, the purified BD enables rapid characterization of aggregation of double helix structures of A-type crystal structure, and screening of compounds which could affect the phenomena for prediction of potentials in starch modification as well.
HPAEC-PAD, High performance anion exchange chromatography-pulsed amperometric detection; BD, branched dextrin; LD, linear dextrin.
Starch is the main carbohydrate source in staple foods such as cereals and root vegetables, as well as versatile ingredient for addition of nutrient values, unique textures, and functionalities in processed foods.1) In various steps in food processing, semi-crystalline native starch is gelatinized to an amorphous form by heating with water to make it readily digestible and/or give it a unique texture.2) Starch gelatinization liberates its two types of α-glucan chains (i.e., amylose and amylopectin) in their water-swollen forms, and these amorphous chains are known to interact with each other and other guest molecules such as fatty acids and aliphatic alcohols.3)
To date, two kinds of chain-chain interactions: (1) double helix formation and association and (2) association of single helices, have been intensively investigated;4)5) the former forms A-type and B-type crystals, and the latter forms V-type crystal. A-type or B-type crystal composes structure of amylopectin in native starch, and mild acid hydrolysis of the starch is used for preparation of insoluble crystals with the same crystalline type as the starch by selective degradation of the amorphous region. The crystalline solid after hydrolysis by sulfuric acid and hydrochloric acid are reported as Nägeli amylodextrin6) and lintnerized starch,7) respectively. Kainuma and French used Nägeli amylodextrin from potato starch for proposal of double helix structure of amylopectin,8) which has been supported by structural analysis data of crystals indicating that both polymorphs are composed of double helices.9)10) Meanwhile, double helix crystal structure is formed during retrogradation of both amylose5) and amylopectin,11)12) indicating that elucidation of double helix formation and association is crucial not only for understanding starch biosynthesis but also for controlling starch properties in foods.
For extraction of focused interactions of starch, model compounds with smaller molecular mass and simpler chemical structures have been developed because multiple types of interactions might simultaneously occur if starch is used for analysis.13) For double helix formation, enzymatically synthesized, linear dextrin (LD) or polymeric amylose without α1,6-linked branches have been used as a model compound for crystal formation experiments.14)15)
Branched dextrin (BD), purified from Nägeli amylodextrin or lintnerized starch, is regarded as a better model of amylopectin than LD and polymeric amylose in terms of the similarity to highly branched amylopectin structure. Ohnishi and French investigated iodine binding property of BD to prove that the branching point in the molecule does not disturb the carbohydrate-iodine complex formation.16) Kitahara et al. recovered BD and LD by fractionation of Nägeli amylodextrin, and their structures and double-helix forming properties were investigated.17) In 16 M methanol, BD developed turbidity more rapidly than LD, suggesting the difference of velocity between intramolecular double helix formation in BD and intermolecular double helix formation by two molecules of LD. They also observed retardation of turbidity development of both kinds of dextrin in the presence of lauric acid.
Purification of BD by a stepwise precipitation using pyridine-methanol-water was reported by Kikumoto and French, which gives us a fraction (Fraction II) rich in BD.18) Meanwhile, Kitahara et al. further purified BD by repeating the fractionation step of Fraction II and recrystallization in 70 % methanol, suggesting the presence of impurities in Fraction II.17) Hall and Manners reported the presence of dextrin with two branch points in a purified BD fraction from Nägeli amylodextrin by a repeated gel filtration.19) According to our estimation of Fraction II from waxy rice starch, the purity of BD in Fraction II was around 78.5 %, and the rest 21.5 % was composed of LD (this study). In this study we focused on further purification of BD in Fraction II by its separation from LD in ethanol solution to observe the velocities of double helix formation before and after purification.
Materials. Waxy rice starch (Mochiru B) was obtained from Joetsu Starch Co., Ltd. (Niigata, Japan). Isoamylase from Pseudomonas sp. was purchased from Megazyme International Ireland (Wicklow, Ireland). Highly branched cyclic dextrin (HBCD, Cluster DextrinTM) was obtained from Ezaki Glico Co., Ltd. (Osaka, Japan). All other chemicals were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Preparation of linear- and branched dextrins from Nägeli amylodextrin. Linear and branched dextrins were prepared according to the method by Kikumoto and French18) with modification: for preparation of Nägeli amylodextrin, 100 g of waxy rice starch was suspended in 2 L of 16 % H2SO4 and incubated at 37 °C with agitation at 60 rpm for 35 days; the amylodextrin was recovered by centrifuging at 5,000 × G for 10 min at 25 °C and repeated washing with water until neutralized; amylodextrin was separated into fractions I, II, and III using pyridine-methanol-water.
Preparation of branched dextrin (BD). Amylodextrin Fraction II was dissolved in hot water, cooled to room temperature, then water and ethanol was added to final concentrations of 100 g/L dextrin and 0 to 60 % ethanol. The mixture was left still at 4, 25, or 40 °C for 1 day for the precipitate to form. The precipitate was collected by centrifuging at 15,000 × G for 5 min, washed with water and air dried to weigh for calculating yield.
Analysis of chain length distribution. The dextrins were dissolved in hot water and analyzed with high performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD). The HPAEC-PAD was conducted using a Dionex ICS-5000+ system (Thermo Scientific, Waltham, MA, USA) on a CarboPac PA-1 column at 1 mL/min elution with a linear gradient of 50 to 500 mM sodium acetate in 50 min in 100 mM sodium hydroxide. For isoamylase debranching, 1 mg of dextrin was dissolved in 100 mM sodium acetate buffer pH 4.0 containing 0.02 % sodium azide and hydrolyzed by adding 5 µL of isoamylase (300 units) and incubating at 40 °C for 24 h. The hydrolyzed sample solution was filtered and analyzed with HPAEC-PAD.
Aggregation experiment in 16 M methanol. The dextrins were dissolved in water and heated at 100 °C for 10 min, then the solution was adjusted to 25 mg/mL by adding water or additive solution. Using 96-well plastic plate, 100 µL of dextrin solution was put in each well and 162.5 µL of methanol was added so the final methanol concentration was approximately 16 M (65 % (v/v)). Absorbance at 660 nm was recorded using a microplate reader (Spectra max plus 384, Molecular Devices, San Jose, CA, USA) at 25 °C for 60 min.
X-ray diffraction analysis. The moisture content of the dextrin sample was adjusted by putting the sample in a chamber with saturated KCl at 23 °C (at 85 % relative humidity) for one month. The samples were sealed in a capillary to prevent moisture changes. The X-ray diffraction analysis was performed on an X-ray diffractometer (Rigaku R-axis Rapid II) with Cu Kα radiation set at 50 kV and 100 mA and exposed for 2400 s.
All experiments were replicated, and average values are presented.
Purification of branched dextrin.
From 100 g of waxy rice starch, an average of 15 g of Nägeli amylodextrin was collected. The average yield of Fractions I, II, and III from Nägeli amylodextrin were 14, 56, and 24 %, respectively. Figure 1A indicates chain length distribution of Fraction II, and there are two trends of distribution in the chromatogram. Isoamylase debranching of the chains resulted in convergence to a single trend of chain length distribution by HPAEC-PAD analysis (Fig. 1B), suggesting that the left trend represents linear dextrin (LD) and right trend represents branched dextrin (BD). In this study, we defined LD and BD as the fraction eluted from 20 to 32 min and that from 32 to 50 min as shown in Fig. 1A, respectively. Former studies indicate that BD fraction is mainly composed of singly-branched chains17)18)19) whereas the presence of short-chain branches in isoamylase-treated Fraction II should be considered because of substrate specificity of isoamylase.20) Herein, we assume that each dextrin elutes following the order of its degree of polymerization (d.p.), and the mode of d.p. of LD and BD are estimated as 13 and 25, respectively. We also calculated the peak area percentage of LD or BD according to molecular mass of each peak, and the ratio of BD in Fraction II was 78.5 % in this study. Practically, the yield of BD in Fraction II is affected by various factors in feedstock and acid hydrolysis. Contamination of LD in Fraction II might be attributed that the purification of Fraction II is a recrystallization-based process;18) although the difference of velocity of association is distinct between LD and BD,17) double helices of both types of dextrin would associate heterogeneously to form crystals of Fraction II.

Next, further recrystallization process with ethanol solution from 100 g/L dextrin was performed for BD purification. Figure 2 shows the effect of recrystallization condition on recoveries of LD and BD. It was found that the lower temperature improves the recovery of dextrin at lower ethanol concentration. We also tested methanol solution for our preliminary experiments to determine the optimum condition to be 10 % (v/v) methanol at 4 °C (data not shown). Because the recovery of BD as well as the purity of BD should be considered, we selected a purification condition of 10 % (v/v) ethanol at 4 °C for further experiments.

A, 4 °C; B, 25 °C; C, 40 °C.
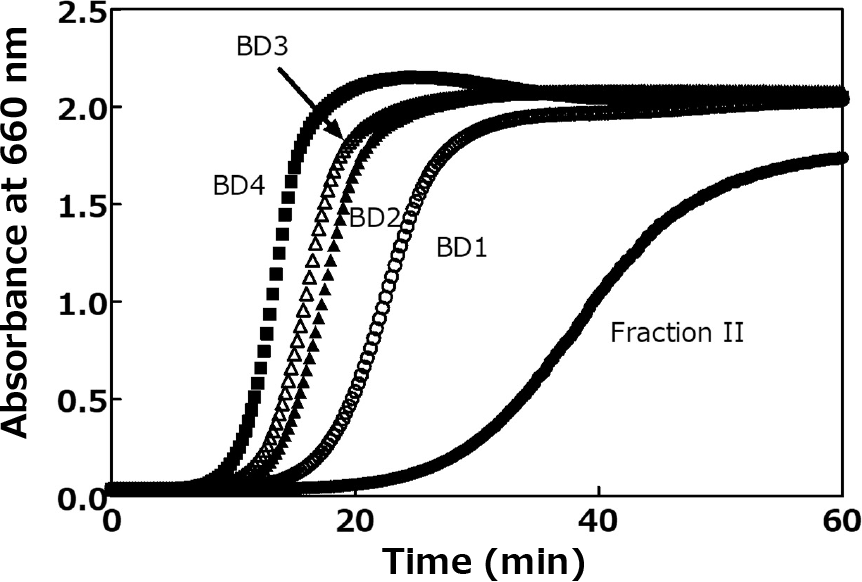
Figure 3 indicates the effect of repeated purification steps with ethanol solution on recovery and purity of BD. The recovery of BD at the first recrystallization of Fraction II was 56.5 %, whereas the purity of BD increased from 78.5 to 88.1 %. As for the second to fourth recrystallization steps, BD purity increased to 94.5 %, suggesting that BD molecules with the higher recrystallizing property would be successfully recovered as the purification step proceeds. The chain length distributions of repeatedly purified samples were shown in Fig. 4; the proportion of LD in the sample decreased as the purification proceeded. We term these four kinds of BD samples after ×1, ×2, ×3, and ×4 repeating purification BD1, BD2, BD3, and BD4, respectively.

Closed bar, BD; open bar, LD.

×n indicate number of repeated purifications in 10 % ethanol at 4 °C.
Association of BD in 16 M methanol.
Then, we investigated turbidity development of each BD sample in 16 M methanol solution, by a modified method of Kitahara et al.;17) a spectrophotometer for 96-well plastic plates was adopted instead of a photoelectric colorimeter for high-throughput analysis. The turbidity development of Fraction II solution was distinct after 20 min of reaction, whereas BD samples after repeated purification in ethanol solution associate more rapidly, and the development of BD4 solution started after 6 min of reaction (Fig. 5). It would be due to a difference in concentration of BD in solutions of Fraction II and the other BDs, because velocity of association of BD depends on concentration.17) The other speculation is that a small amount of LD would interact with BD to retard its association. Lauric acid retarded the association of BD, suggesting that BD would form an inclusion complex with lauric acid, which would be released during the association of BD.17) As for LD, the possible interaction with BD would not be inclusion complex formation like lauric acid but double helix formation or other chain-chain interactions; Xu et al. reported that spring dextrin, defined as a series of linear dextrin from amylose, retards amylose-amylose interaction.21)
The crystal structure of BD4 crystallized in 10 % ethanol was estimated as B-type by X-ray diffractometry (Fig. 6B), whereas the same BD4 crystallized in 16 M methanol was A-type (Fig. 6A). Previous structural data showed that the type of crystal structure of recrystallized amylodextrin depended on chain-length, temperature, concentration, the presence of salts, and the presence of water-soluble alcohols and organic acids.22)23) It is reasonable that BD crystallized in 10 % ethanol and 16 M methanol showed different crystalline type. Choice of solvent to be used in an assay method for crystal formation is critical, and 16 M methanol can be used in an assay for formation of A-type crystal.

Thus, we expect that the purified BD would have the higher quality with less impurities than Fraction II as a new tool for characterization of double helix formation and its association to form A-type crystalline flakes.17) We hypothesize that at least three steps of reactions would be needed for crystalline flake formation from BD: double intramolecular helix formation, association of the helices to organize a nucleus of crystal, and growth and/or association of crystals to form flakes. The assay system of turbidity development in methanol solution would be applied not only for basic analysis of A-type crystal development but also for screening of compounds or factors affecting the crystal development, which would be potentially used for control of starch structures and functions as well as design of novel materials based on double helix structure.24)25)
Effect of additives on BD association.
We used highly branched cyclic dextrin (HBCD) and sodium tetraborate, which are known to interact with gelatinized starch,26)27) to estimate the effect of each compound on double helix formation and association by the turbidity development assay system using BD4. The addition of HBCD resulted in retardation of BD4 flake formation and the decrease of the turbidity at the plateau stage (Fig. 7). HBCD retards turbidity development of soluble starch solution in water,26) and the same trend was observed in this study. In Fig. 7, the curves at the initial increases of turbidity seem to be distinct with each other, implying that HBCD would retard a certain structure of BD at the lag phase to reach minimum necessary concentration to start association for raising the turbidity. Meanwhile, the effect of sodium tetraborate on association of BD4 was not clearly observed, except for a slight shift of the turbidity at the plateau stage (Fig. 8). Sodium tetraborate is known to interact with gelatinized starch to decrease the critical concentration of starch gelation.27) The ready-to-associate structure of BD for raising the turbidity might reach the minimum necessary concentration at the same timing in all samples in Fig. 8. Also, careful interpretation of the difference in the turbidity at the plateau stage in Fig. 8 will be needed, because it might be influenced not only by the number but also by the size of the flakes.28) In this study, we prepared purified BD samples and succeeded in analysis of the association and turbidity increase more sensitively than before, whereas quantitative characterization of the phenomena during BD association will be needed for deeper understanding and more precise controlling of starch and its related compounds.

It should be noted that BD4 obtained at different purification occasion was used (with 98 % purity) for obtaining the turbidity curve for Figs. 7 and 8.

It should be noted that BD4 obtained at different purification occasion was used (with 98 % purity) for obtaining the turbidity curve for Figs. 7 and 8.
In this study, we found that BD prepared by further purification of Fraction II from Nägeli amylodextrin shows improved association property in 16 M methanol, and a rapid, small-scale assay system for crystal formation by double helices was established as a modified method of previous one.17) This assay system works as a simple model of association of double helices to develop A-type crystal flakes by separation from other interactions occurring in starch, whereas care must be taken because methanol might affect the interaction between BD and additives. Further understanding of basic phenomena on A-type crystal formation and its interaction with various additives would give us information for regulation of structure and function of starch and its derivatives.
The authors declare no conflicts of interest.
This work was supported in part by Cabinet Office, Government of Japan, Cross-ministerial Strategic Innovation Promotion Program (SIP), “Technologies for Smart Bio-industry and Agriculture” (funding agency: Bio-oriented Technology Research Advancement Institution, NARO). The authors would like to thank Ms. Nobuko Yoshii and Ms. Junko Nakatsuji for their great technical contributions in this study.