2020 Volume 67 Issue 1 Pages 11-15
2020 Volume 67 Issue 1 Pages 11-15
Glucose and fructose were treated in subcritical water in the presence of alkali or alkaline earth metal chlorides. All salts accelerated the conversion of saccharides, and alkaline earth metal chloride greatly promoted the isomerization of glucose to fructose. In contrast, alkali metal salts only slightly promoted this isomerization and facilitated the decomposition of glucose to byproducts such as organic acids. The selectivity of the glucose-to-fructose isomerization was higher at lower conversions of glucose and in the presence of alkaline earth metal chlorides. The pH of the reaction mixture also greatly affected the selectivity, which decreased rapidly at lower pH due to the generated organic acids. At low pH, decomposition of glucose became dominant over isomerization, but further conversion of glucose was suppressed. This result was elucidated by the suppression of the alkali-induced isomerization of glucose at low pH. Fructose underwent decomposition during the treatment of the fructose solution, but its isomerization to glucose was not observed. The added salts autocatalytically promoted the decomposition of fructose, and the reaction mechanism of fructose decomposition differed from that of glucose.
Recently, subcritical water has been used in food processing. Subcritical water maintains its liquid state under pressurized conditions at a high temperature (100–374 °C). Subcritical water exhibits two distinct properties: a low dielectric constant and a high ion product.1) The low dielectric constant facilitates the extraction of hydrophobic components from biomass, whereas the high ion product indicates that subcritical water contains hydronium and hydroxide ions at high concentrations. Because of these properties, subcritical water can act as a base or an acid catalyst and promote numerous reactions.
During food processing, many reactions occur in the food constituents, including saccharides and proteins, in addition to their extraction.2)3) A major reaction of saccharides in subcritical water is the hydrolysis of oligo- and polysaccharides to form monosaccharides.4)5)6)7) Reducing monosaccharides can further react in subcritical water, isomerizing to form other types of reducing saccharides.8)9)10) For example, glucose can isomerize to form fructose and mannose through enediol-structured intermediates via Lobry de Bruyn-Alberda-van Ekenstein (LBAE) transformation under basic conditions.8)11)12)13)14) In addition, saccharides can decompose in parallel with the isomerization, resulting in the formation of byproducts such as organic acids.15)
Salts are often used during food processing, and previous reports have investigated the effects of salt on saccharide reactions in subcritical water.14)16)17) Although the salts accelerated the decomposition of saccharides in subcritical water, these reports mainly dealt with the effects of sodium chloride, which is insufficient to elucidate the effects of different salts on the reaction behavior. Herein, the effects of the salt type (i.e., alkali and alkaline earth metal chlorides) on the isomerization and side reactions (decomposition) of glucose were evaluated in subcritical water. The reactions of fructose were also quantitatively evaluated under the same conditions.
Materials. Glucose, fructose, and the tested salts (LiCl, NaCl, KCl, CsCl, MgCl2, CaCl2, and SrCl2) were purchased from FUJIFILM Wako Pure Chemical (Osaka, Japan). Other chemicals were obtained from FUJIFILM Wako or Nacalai Tesque (Kyoto, Japan).
Subcritical water treatment of glucose and fructose. Subcritical water treatment of the saccharides was performed using a tubular reactor, in a similar manner to a previous report.16) The reactor consisted of an HPLC pump (LC-10ADVP, Shimadzu, Kyoto, Japan) connected to PEEK tubing (0.8 mm I.D. × 4.0 m), which was immersed in an oil bath thermostatted at 190 °C. The outlet side of the reactor was immersed in a water bath to rapidly terminate the reaction. The pressure inside the reactor was maintained at ca. 6 MPa using a back-pressure regulator (P-880, Upchurch Scientific, Oak Harbor, WA, USA). Although the PEEK tubing was inert against salt, its pressure resistance was low at high temperatures. Therefore, to avoid breakage of the PEEK tubing, it was inserted in a stainless-steel tube (2 mm I.D.).
Glucose or fructose was dissolved in distilled water to a final concentration of 5 % (g/g-solution). Salt was added to this solution to a final concentration of 0.50 mol/L to prepare the starting mixture. Nitrogen gas was then sufficiently bubbled into the mixture to remove dissolved oxygen, and its reservoir was connected to a nitrogen gas bag to prevent the redissolution of oxygen. The starting mixture was delivered to the reactor using an HPLC pump to begin the reaction. The flow rate was adjusted to yield residence times (reaction times) of 30–300 s. The effluent from the reactor was collected and analyzed by HPLC. The experiments were performed in n = 4 for NaCl and KCl, and n = 3 for other cases. The pH measurements were performed once.
HPLC analysis. Prior to HPLC analysis, metal ions in the reaction mixture were removed by adsorption. The reaction mixture was mixed with an equal volume of 1 % (g/g-solution) xylose solution in water. The solution was subsequently passed through a column packed with 1 mL of wet DOWEX 50W×8 cation exchange resin (H+ form), and the effluent was subjected to HPLC analysis. Glucose and fructose were quantified using an HPLC system equipped with an RID-20A refractive index detector (Shimadzu) and LC-20AD HPLC pump (Shimadzu) connected to a COSMOSIL Sugar-D column (3 mm I.D. × 250 mm, Nacalai Tesque). The eluent was 80 % acetonitrile at a flow rate of 0.4 mL/min.
Conversion of glucose in the presence of salts.
Figure 1 shows the remaining fraction of glucose during its subcritical water treatment plotted as a function of residence time on a semilogarithmic scale. In subcritical water in the absence of salt (referred to as 'pure subcritical water'), ca. 6 % of the glucose reacted within 120 s. The presence of the alkali metal salt accelerated the conversion of glucose (80–93 % of remaining glucose at 120 s). In the presence of an alkaline earth metal salt, the glucose concentration was further decreased to 69–73 % of the original concentration after a residence time of 120 s.

Symbols (open square, open triangle, open diamond, open reverse triangle, closed circle, closed square, and closed triangle) represent the results obtained in the presence of LiCl, NaCl, KCl, CsCl, MgCl2, CaCl2, and SrCl2, respectively. The open circle symbol represents results obtained from the treatment in pure subcritical water.
All decreasing curves exhibited a downward convex pattern and did not yield straight lines, indicating that the decomposition of saccharides cannot be described by the first-order kinetics. In addition, the decrease in the glucose content slowed when the remaining glucose reached ˂80 % of the original concentration. In particular, the decrease in the glucose content was very slow in the latter half of the reaction in the presence of alkaline earth metal salts.
Effects of salts on the formation of fructose.
Figure 2 shows time courses of fructose formation by the subcritical water treatment of glucose. Under each condition, an increase in the fructose yield was observed during the early period of the reaction (within the first 90 s). In pure subcritical water, ca. 1.9 % fructose was formed, and in the presence of alkali metal salts, fructose formation was slightly faster than that observed in pure subcritical water. However, effect of the salt was not significant, and the highest fructose yield was 2.3 %. In the presence of alkaline earth metal salt, however, fructose yields increased remarkably within 60 s with ˃14 % fructose formed upon addition of MgCl2; ca. 4–7 % fructose was achieved in the presence of CaCl2 or SrCl2.
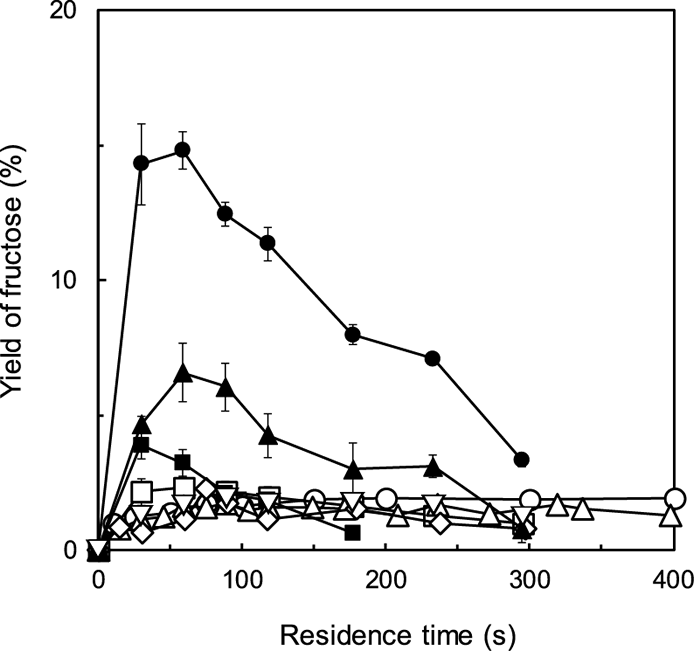
The symbols are the same as those used in Fig. 1.
Although decrease in the fructose content was not observed over the studied period in pure subcritical water, the fructose content began to decrease at 60–90 s upon salt addition. Especially, the presence of alkaline earth metal salt greatly affected the decrease of fructose. This tendency indicates that the cation valence affected the promotion of isomerization and decomposition. It was reported that calcium ion promoted the retro-aldol condensation (α-dicarbonyl cleavage) by coordination of calcium ion at C1–3 oxygen atoms, resulting in the decomposition of the saccharides and formation of organic acids.18) In the case in this study, similar phenomena would occur during the subcritical water treatment when divalent ion was present. This is because the decomposition of the formed fructose was faster than the isomerization of glucose to fructose.
Change of pH during the reaction.
The pH change of the reaction mixture was plotted as a function of the residence time during the conversion of glucose in subcritical water (Fig. 3). The initial pH of the solution depended on the nature of the added salts, reaching a value of pH 6.0 in the pure subcritical water, but was lowered to 4.8–5.0 when an alkali metal salt was added. Upon addition of an alkaline earth metal salt, the initial pH ranged from 6.2 to 8.8. The pH steeply decreased to ˂4 at 30 s during the subcritical water treatment under every treatment condition and finally reached ca. 3.3 in pure subcritical water. Upon addition of the alkali metal salt, the final pH fell to 2.9–3.0. The addition of alkaline earth metal salt resulted in a further decrease of the final pH to ca. 2.3–2.6. The reason for great decrease of the final pH in the presence of alkaline earth metal salts would be due to the formation of organic acids by the presence of divalent ion as above mentioned.

The symbols are the same as those used in Fig. 1.
The pH was related to the variation in the reaction mechanism. Under each treatment condition, the pH sharply decreased during the early reaction stages, which was equivalent to performing the reaction with the gradual addition of acid. It has been reported that each saccharide decomposes in parallel with isomerization.9)12)18) Isomerization of glucose progresses through a mechanism equivalent to alkali isomerization (LBAE transformation) at higher pH. Because this reaction is inhibited under acidic conditions, the consumption of glucose by isomerization slowed with decreasing pH. As a result, the glucose consumption rate decreased during the latter half of the reaction.
Relationship between the selectivity of fructose and reaction behavior.
The selectivity of fructose generation during the glucose treatment was related to the conversion of glucose and decreasing pH (Fig. 4). Here, the selectivity for fructose generation is defined as the amount of generated fructose against that of the consumed glucose. Figure 4A shows the relationship between the selectivity for fructose generation and the conversion of glucose. Under every treatment condition, the selectivity exponentially decreased with increasing glucose conversion.

The symbols are the same as those used in Fig. 1.
The final conversion of glucose in pure subcritical water was 11 % with the fructose selectivity of 20 %. However, upon addition of alkali metal salts, the glucose conversion increased. The final selectivity further fell to ca. 4 %, which was much lower than that obtained in pure subcritical water. In contrast, the selectivity was much higher in the presence of alkaline earth metal salts. In addition, the type of the alkaline earth metal salt greatly affected the selectivity, with MgCl2 showing particularly high selectivity.
Figure 4B shows the selectivity dependence on the pH of the treated solution where the selectivity decreased with decreasing pH. The degree of the pH decrease during the treatment was small in pure subcritical water, and the selectivity decrease was also relatively small. However, upon addition of an alkali metal salt, both the pH and selectivity greatly decreased as the reaction progressed. When an alkaline earth metal salt was added, a similar trend was observed. However, in the presence of an alkaline earth metal salt, the selectivity was higher than that in the presence of alkali metal salts. One of its reasons would be the higher initial pH of the glucose solution. Generally, alkaline earth metal salts contain basic impurities, raising the pH during the initial stages of the reaction, which would promote alkali isomerization and improve the selectivity for fructose generation.
Subcritical water treatment of fructose.
Fructose was also treated in subcritical water in the presence of salts. Time courses showing the remaining fructose are provided on a semilogarithmic scale in Fig. 5. No time course yielded a straight line and instead showed an upward convex pattern, indicating that the decrease in the fructose content accelerated as the reaction progressed and that the decomposition of saccharides cannot be described by the first-order kinetics. As described above, when glucose was treated in subcritical water, its conversion slowed in the latter half of the reaction. In contrast, the conversion behavior of fructose was completely different from that of glucose.

The symbols are the same as those used in Fig. 1.
When fructose was treated in subcritical water in the presence of salt, its decomposition was accelerated compared to that observed in pure subcritical water. The reaction was greatly accelerated when alkaline earth metal salts were added, but the nature of the alkaline earth metal salt hardly influenced the reaction behavior. Only 30–50 % of the original fructose content remained after 90 s in the presence of alkaline earth metal salts, whereas ˃ 65 % of the glucose remained during its treatment under the same conditions (Fig. 1). Therefore, fructose was decomposed more rapidly than glucose under the conditions tested herein.
Figure 6 shows time courses of the pH change during the decomposition of fructose. When alkaline earth metal salts were added, the initial pH was relatively high (6.6–8.2). However, the pH decreased to < 4 after 30 s of the reaction under every condition tested. The decrease in pH was remarkable in the presence of alkaline earth metal salts, reaching ˂ 3 after 30 s of the reaction. Further decrease in pH over time was more remarkable in the presence of salts than that observed in pure subcritical water. The type of cation of the alkali or alkaline earth metal salts did not significantly influence the pH.

The symbols are the same as those used in Fig. 1.
Fructose also decomposes or isomerizes via LBAE transformation,18)19) but isomerization to glucose was not observed herein because fructose would be thermodynamically more stable than glucose at higher temperatures. Meanwhile, fructose is prone to decomposition in the presence of acids. As the reaction progressed, the acid-catalyzed decomposition dominated, resulting in the further formation of organic acids. As a result, isomerization was completely inhibited and fructose decomposition accelerated in the latter half of the reaction. It has been reported that the acid-catalyzed decomposition of fructose autocatalytically progresses in the presence of hydronium ions,20)21) supporting the acceleration observed in this study. Meanwhile, changes in the reaction mechanism were not observed upon the addition of salts. From the results presented herein, salts only promoted the existing reactions and did not change the underlying mechanism.
Glucose and fructose were treated in subcritical water in the presence of various salts. Alkali metal salts promoted the decomposition of saccharides, whereas alkaline earth metal salt greatly promoted the isomerization of glucose to fructose. The selectivity for the generation of fructose during isomerization was higher at lower glucose conversions and higher pH of the reaction mixture. With the progression of the reaction of glucose, its decomposition became dominant over isomerization, and the selectivity of fructose generation was decreased at lower pH. In an analogous treatment of fructose, only decomposition was observed, and the reaction mechanisms differed from those of glucose.
The authors declare no conflict of interests.
This study was financially supported by Salt Science Research Foundation (No.1654; T.K.) and JSPS KAKENHI (No. 17K07814; T.K.).