2021 Volume 68 Issue 3 Pages 53-61
2021 Volume 68 Issue 3 Pages 53-61
Carbohydrate materials that produce lower postprandial blood glucose increase are required for diabetic patients. To develop slowly digestible carbohydrates, the effect of degree of polymerization (DP) of α-1,6 glucan on its digestibility was investigated in vitro and in vivo. We prepared four fractions of α-1,6 glucan composed primarily of DP 3–9, DP 10–30, DP 31–150, and DP 151+ by fractionating a dextran hydrolysate. An in vitro experiment using digestive enzymes showed that the glucose productions of DP 3–9, DP 10–30, DP 31–150, and DP 151+ were 70.3, 53.4, 28.2, and 19.2 % in 2 h, and 92.1, 83.9, 39.6, and 33.3 % in 24 h relative to dextrin, respectively. An in vivo glycemic response showed that the incremental area under the curve (iAUC) of blood glucose levels of α-1,6 glucan with DP 3–9, DP 10–30, DP 31–150, and DP 151+ were 99.5, 84.3, 65.4, and 40.1 % relative to dextrin, respectively. These results indicated that α-1,6 glucan with higher DP had stronger resistance to digestion and produced a smaller blood glucose response. DP 10–30 showed significantly lower maximum blood glucose levels than dextrin; however, no significant difference was observed in iAUC, indicating that DP 10–30 was slowly digestible. In addition, α-1,6 glucan was also produced using an enzymatic reaction with dextrin dextranase (DDase). This produced similar results to DP 10–30. The DDase product can be synthesized from dextrin at low cost. This glucan is expected to be useful as a slowly digestible carbohydrate source.
DP, degree of polymerization; DDase, dextrin dextranase; HPLC, high-performance liquid chromatography; iCmax, incremental maximum concentration of blood glucose; iAUC, incremental area under the curve.
Elderly people sometimes require nutritional support with oral nutritional supplements (ONS) or tube feeding with a liquid diet due to undernutrition or dysphagia. The typical ONS is a liquid diet containing the starch degradation product dextrin as carbohydrate source.1) Because dextrin is rapidly digested and absorbed in the human digestive tract, it tends to cause high blood glucose levels after ingestion, especially in people with impaired glucose tolerance. The number of elderly diabetic patients is increasing in developed countries,2) and high blood glucose levels may cause negative effects on their clinical conditions. Thus, carbohydrate materials that produce lower postprandial blood glucose levels than dextrin are needed.
Blood glucose levels after ingestion of α-glucans, such as starch and dextrin, are strongly related to digestibility in the small intestine.3) The blood glucose increase will be attenuated if carbohydrates are digested slowly. However, if carbohydrates are digested too slowly or not digested, sufficient energy cannot be obtained from their ingestion. Therefore, a slowly digestible carbohydrate that can be completely digested in the small intestine is useful as a carbohydrate energy source for elderly diabetic patients. By adding a slowly digestible carbohydrate to liquid diets instead of dextrin, it is possible to provide sufficient energy without rapid blood glucose increase after ingestion. The digestibility of glucan is related to the frequency of branched structures, glycosidic bond types and crystal structures.4)5)6) α-1,4 glycosidic bonds are the main bonds in starch and dextrin structures and are readily hydrolyzed by digestive enzymes, whereas α-1,6 glycosidic bonds present in branched structures are hydrolyzed slowly.7)
In previous studies, the digestibility and blood glucose responses of glucans with α-1,6 glycosidic bonds were investigated for the purpose of developing slowly digestible carbohydrates.8)9) These studies suggest that the position of α-1,6 glycosidic bonds is more important than their ratio to α-1,4 glycosidic bonds in suppressing in vivo blood glucose increases. Moreover, α-1,6 glycosidic bonds at non-reducing ends are more effective than α-1,6 glycosidic bonds in the inner regions of the glucose chain.
It is expected that a linear glucan consisting almost entirely of α-1,6 glycosidic bonds, including at the non-reducing end (called α-1,6 glucan), would attenuate postprandial blood glucose levels more effectively as DP increases. Thus, α-1,6 glucan in a particular DP range may represent a slowly digestible glucan. However, few studies have examined the relationship between α-1,6 glucan DP, its digestibility and blood glucose suppression.
There are two methods to prepare α-1,6 glucan: hydrolysis of dextran and synthesis from a starch hydrolysate like a dextrin using dextrin dextranase (DDase). DDase degrades α-1,4 glycosidic bonds at non-reducing ends and produces α-1,6 glycosidic bonds at other non-reducing ends.10) These reactions occur repeatedly and result in continuous α-1,6 glucan structures at non-reducing ends. The glucan obtained by dextran degradation consists mainly of α-1,6 glycosidic bonds, whereas the glucan produced by the DDase reaction includes α-1,4 glycosidic bonds at reducing ends.10) Enzymatic synthesis using DDase is more suitable for industrial applications because of its low cost.
The purpose of this study is to evaluate the effects of DP and α-1,6 glucan synthesis method on its digestibility and effects on blood glucose. First, hydrolyzed dextran was fractionated according to specified DP ranges and the relationship between DP of α-1,6 glucan, digestibility and blood glucose increase was evaluated. Second, α-1,6 glucan from dextrin was prepared with a DDase reaction, and its digestibility and blood glucose effects were evaluated.
Materials. Dextran 70 was purchased from Meito Sangyo Co., Ltd. (Aichi, Japan). TOYOPEARL HW40C was purchased from Tosoh Corporation (Tokyo, Japan). Amberlite MB4 was purchased from Organo Corporation (Tokyo, Japan). Other chemicals were purchased from Kanto Chemical Co., Inc. (Tokyo, Japan).
Preparation of DP 1–30 α-1,6 glucan from dextran. Dextran 70 was dissolved at 25 % (w/w) in ultrapure water, hydrochloric acid was added at a final concentration of 45 mM and the sample was heated to 80 °C for 53 h. After the DP ≥ 31 components were almost completely degraded, the reaction solution was cooled to room temperature. This solution was mixed with MB4 for desalination, filtered with a 0.45 μm pore diameter filter, and lyophilized. The resultant α-1,6 glucan consisted primarily of DP 1–30.
Preparation of DP 3–9 α-1,6 glucan from hydrolyzed dextran. A 20 % (w/w) aqueous solution of DP 1–30 α-1,6 glucan, from above, was heated to 50 °C for 10 min and ethanol was added with stirring to a final 70 % (v/v) concentration. After the solution was incubated at 4 °C for 3 h, the supernatant and precipitate were separated by decantation, and it was confirmed that the supernatant fraction did not contain DP ≥ 10. The precipitate was re-dissolved in water, and the precipitation/separation procedure was repeated three times to isolate the DP 3–9 fraction from the precipitate. The resultant supernatant fraction was concentrated by evaporation and applied to gel filtration chromatography (TOYOPEARL HW40C) to further purify the DP 3–9 fraction. The resulting solution was desalted by MB4 and filtered as above and subsequently lyophilized. The resultant α-1,6 glucan (DP 3–9) comprised DP 3–9 as the main component.
Preparation of DP 10–30 α-1,6 glucan from hydrolyzed dextran. The ethanol precipitate obtained in the preparation of DP 3–9 was applied to gel filtration chromatography (TOYOPEARL HW40C) to purify the DP 10–30 fraction. The obtained solution was desalted by MB4 and filtered as above, and subsequently lyophilized. The resultant α-1,6 glucan (DP 10–30) comprised DP 10–30 as the main component.
Preparation of DP 31–150 α-1,6 glucan from dextran. Dextran 70 was dissolved at 10 % (w/w) in ultrapure water, dextranase from Chaetomium erraticum (Dextranase L “Amano”; Amano Enzyme Inc., Aichi, Japan) was added at 1.2 U/g-substrate and the reaction was conducted at 60 °C and pH 6.0 for 24 h. After the DP ≥ 150 components were almost completely hydrolyzed, the reaction mixture was incubated at 80 °C for 12 h at pH 4.0 to stop the reaction. The obtained solution was desalted by MB4 and filtered as above. The resultant α-1,6 glucan comprised DP ≤ 150 as the main component. A 20 % (w/w) aqueous solution of this α-1,6 glucan was heated to 50 °C and ethanol was added with stirring to obtain a 55 % (v/v) final solution. After incubation at 4 °C for 3 h, the supernatant was removed by decantation. The precipitate was re-dissolved in water and the precipitation/separation procedure was repeated until the DP ≤ 30 components were mostly removed. The obtained target product was dissolved in water and lyophilized. The resultant α-1,6 glucan (DP 31–150) comprised DP 31–150 as the main component.
Preparation of DP 151+ α-1,6 glucan from dextran. Dextran 70 was dissolved at 20 % (w/w) in ultrapure water. The solution was passed through an ultrafiltration membrane (NMWL 30,000, Regenerated cellulose, Millipore Corporation, Bedford, MA, USA) under pressure condition using CO2 gas to remove DP ≤ 150. Ultrapure water was added to maintain the solution volume following ultrafiltration. This operation was repeated until the DP > 151 component of the solution became the main component. The obtained α-1,6 glucan (DP 151+) was subsequently lyophilized.
Preparation of DDase. Gluconobacter oxydans ATCC 11894 was cultured in a medium containing 10 % (w/w) sucrose (Mitsui Sugar Co., Ltd., Tokyo, Japan), 10 % (w/w) yeast extract (Becton Dickinson and Company, Franklin Lake, NJ, USA) and 0.2 % (w/w) RYOTO Sugar Ester O-170 (Mitsubishi-Chemical Foods Corporation, Tokyo, Japan) for 48 h at pH 6.0 and 26 °C with 180 rpm shaking. The cell-free culture broth was obtained by centrifugation at 5,000 × G for 10 min. The obtained broth was filtered with 0.45 μm pore diameter membrane filter (Advantec Co., Ltd., Tokyo, Japan) and concentrated using an ultrafiltration membrane (Microza AHP-1010D, Asahi Kasei Corporation, Tokyo, Japan).
DDase activity assay. A DDase enzyme solution (0.5 mL) diluted with ultrapure water was mixed with 0.5 mL of 2 % (w/v) maltotetraose dissolved in 50 mM acetate buffer (pH 4.2), and the solution was incubated at 35 °C. Samples (0.5 mL) of the reaction solution were collected at 5 and 60 min after the start of the reaction and incubated at 100 °C for 10 min to stop the reaction. The amount of maltotriose in the solutions were measured by high-performance liquid chromatography (HPLC). One unit (U) of enzyme activity was defined as the amount of the enzyme producing 1 μmol of maltotriose in 1 min. HPLC conditions were as follows: two Ultron PS-80 N.L columns (8.0 × 500 mm; Shinwa Chemical Industries, Kyoto, Japan) were connected in series; column temperature, 80 °C; flow rate, 0.6 mL/min; eluent, ultrapure water; detector, refractive index detector (Chromaster 5450; Hitachi High-Tech Science Corporation, Tokyo, Japan).
Preparation of α-1,6 glucan by DDase. Dextrin (Pinedex #1; Matsutani Chemical Industry, Hyogo, Japan) was dissolved at 30 % (w/w) in ultrapure water, and DDase, α-amylase from Bacillus subtilis (Kleistase L-1; Amano Enzyme), isoamylase from Flavobacterium odoratum (GODO-FIA; GODO SHUSEI CO., LTD., Tokyo, Japan) and pullulanase from Klebsiella pneumoniae (Pullulanase “Amano” 3; Amano Enzyme) were added to the solution at 7, 0.1, 200, and 0.6 U/g-substrate, respectively. The mixture was incubated at 53 °C and pH 5.0 for 72 h. The reaction mixture was then heated to 80 °C for 12 h at pH 4.0 to stop the reaction. The obtained product (DDase product) was desalted by MB4 and filtered as above and lyophilized.
Preparation of α-1,6 glucan by DDase lacking DP 1–2. The above α-1,6 glucan produced with DDase was applied to gel filtration chromatography (TOYOPEARL HW40C) to remove the DP 1–2 fraction. The obtained solution was desalted by MB4 and filtered as above and lyophilized. The resultant α-1,6 glucan (DDase product (–DP 1–2)) lacked the DP 1–2 component.
Analysis of saccharide composition. Saccharide composition was analyzed by HPLC. HPLC conditions for the DP 1–30 fraction were as follows: column, MCL GEL CK02AS (20 × 250 mm; Mitsubishi Chemical Corporation, Tokyo, Japan); column temperature, 80 °C; flow rate, 0.7 mL/min; eluent, ultrapure water; detector, refractive index detector. The elution time for each DP was calculated using a dextrin calibration curve. HPLC conditions for the DP > 30 fraction were as follows: two OHpak SB-806M HQ columns (8.0 × 300 mm; Showa Denko K.K., Tokyo, Japan) connected in series; column temperature, 50 °C; flow rate, 0.3 mL/min; eluent, 50 mM sodium nitrate solution; detector, refractive index detector. The elution time of each DP was calculated using a calibration curve of pullulan standards.
Analysis of the glycosidic bond. Glycosidic bond formulation was calculated from the peak area in 1H NMR. NMR was measured with a Bruker 400 MHz NMR spectrometer (Bruker Japan K.K., Kanagawa, Japan) at room temperature in deuterated water.
In vitro digestibility. Enzymes used in this experiment were prepared as follows. Two grams of a rat small intestine acetone powder (Sigma-Aldrich Japan K.K., Tokyo, Japan) was suspended in 20 mL of 45 mM maleate buffer (pH 6.6). The suspension was centrifuged (19,000 × G, 10 min) to collect the supernatant. The enzyme activity of the resultant solution was measured as maltase activity. Forty microliters of 0.1 M acetate buffer (pH 6.0) and 15 μL of the enzyme solution diluted with ultrapure water were mixed, and the solution was maintained at 37 °C until use. Forty-five microliters of a 2 % (w/v) maltose solution was added to the solution to start the reaction. The reaction was conducted for 10 min, and 200 μL of 2 M Tris-HCl buffer (pH 7.0) was subsequently added to the solution to stop the reaction, and 80 μL of Glucose C-II Test Wako reagent (Wako Pure Chemical Industries, Osaka, Japan) was added. The solution was incubated at 37 °C for 30 min. Absorbance (λ = 490 nm) was measured and the amount of free glucose was calculated based on a glucose standard curve. For enzyme activity, 1 U was defined as the amount of the enzyme producing 2 μmol of glucose in 1 min under the above conditions.
A sample was dissolved at a final concentration of 0.45 % (w/v) in 45 mM maleate buffer (pH 6.6). Porcine pancreatic α-amylase (Type I-A, Sigma-Aldrich Japan) and the enzyme prepared above were added to the sample solution at 4,000 and 860 U/g-substrate, respectively, and the solution was incubated at 37 °C. Twenty microliters of the reaction solution was mixed with 200 μL of 2 M Tris-HCl solution to stop the reaction at predetermined reaction durations. The amount of glucose at each reaction duration was measured using Glucose C-II Test Wako. Dextrin (Pinedex #2; Matsutani Chemical Industry) was used as a control carbohydrate.
In vivo evaluation of postprandial blood glucose. Animals were treated in accordance with the Standards Relating to the Care and Keeping and Reducing Pain of Laboratory Animals in Japan (Notice of the Ministry of the Environment of Japan, No. 84 of 2013) and the Laboratory Protocol of Animal Handling of Morinaga Milk Industry Co., Ltd. All animal experiments were approved by the Institutional Animal Care and Use Committee of Morinaga Milk Industry Co., Ltd. (Approval numbers: 13–052 and 14–033), and performed from Oct. 2013 to Oct. 2014. Five-week-old male SD rats were obtained from Japan SLC Co., Inc. (Shizuoka, Japan). All rats were housed individually in plastic cages under conditions maintained at 22 ± 2 °C with a 12-h light/12-h dark cycle (light on from 8:00 until 20:00). The rats were acclimatized to the laboratory environment until the experiments were initiated and allowed free access to water and regular solid chow (F2, Funabashi Farm Co., Ltd., Chiba, Japan). At seven weeks of age, approximately two-thirds of the rats were selected based on blood glucose levels. Briefly, rats were fasted overnight for 18 h, and blood glucose from tail vain samples was measured using self-monitoring blood glucose devices (Glutest Neo, Sanwa Kagaku Kenkyusho Co., Ltd., Aichi, Japan) at 0, 30, 60, 90 and 120 min after oral administration of 3 g/kg body weight (0.3 g/mL) of dextrin. Animals with a good increase in postprandial blood glucose level were selected. To evaluate α-1,6 glucan production from dextran, 18 rats were purchased. DP 31–150, DP 10–30, DP 3–9, and DP 151+ were evaluated at 8, 9, 10, and 11 weeks of age, respectively. One-week intervals were used as washouts between each test. In each test, rats were fasted overnight for 18 h, and assigned to one of two groups (n = 5–6 each) based on their body weight. Blood glucose after the administration of dextrin or one of the α-1,6 glucan samples was measured using the procedure described above. The samples were prepared as a 0.3 g/mL solution dissolved in distilled water and administered with a feeding needle at a dose of 3 g/kg body weight. In the evaluation of DP 151+ at 11 weeks of age, one animal from the DP 151+ group was excluded due to failure of administration. Free feeding of standard chow was resumed immediately after blood glucose was measured at 120 min. In the evaluation of the DDase product, 24 rats were purchased. Eight-week-old rats were fasted overnight for 18 h and assigned to one of three groups (n = 6 each). Blood glucose after administration of dextrin (control), DDase product or DDase product (–DP 1–2) was measured using the procedure described above. Incremental maximum concentration of blood glucose (iCmax) values were calculated by subtracting the initial blood glucose levels from the maximum blood glucose levels after administration. Incremental area under the curve (iAUC) values were obtained by calculation of the area under the blood glucose curve, with the area beneath the fasting concentration omitted.
Statistical analysis. Data are presented as the mean ± standard error of mean (SEM). Student’s t-test was used for comparisons between two groups. Dunnett’s test was used for multiple comparisons where the control group is compared with experimental groups. Results with p-values < 0.05 were considered significant.
Preparation of a-1,6 glucan from dextran.
Each α-1,6 glucan, with DP 3–9, DP 10–30, DP 31–150 or DP 151+ as the main component, was fractionated from acid or enzymatically hydrolyzed dextran by ethanol precipitation, gel filtration chromatography or membrane separation. The composition of each product was analyzed by HPLC and glycosidic bond content was measured by 1H NMR (Table 1). All the products contained ≥ 80 % of the targeted fractions and the proportions of α-1,6 glycosidic bonds were ≥ 90 %.
| Product | Composition (%) | α-1,6 bond (%) |
||||
|---|---|---|---|---|---|---|
| DP 1–2 | DP 3–9 | DP 10–30 | DP 31–150 | DP 151+ | ||
| DP 3–9 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 98.2 |
| DP 10–30 | 0.0 | 3.1 | 96.9 | 0.0 | 0.0 | 98.2 |
| DP 31–150 | 0.0 | 0.0 | 0.0 | 82.7 | 17.3 | 94.3 |
| DP 151+ | 0.0 | 0.0 | 0.0 | 13.1 | 86.9 | 94.5 |
In vitro digestibility of α-1,6 glucan from dextran.
Digestibility of α-1,6 glucan from dextran is shown in Fig. 1. Rat digestive enzyme and α-amylase were added to the sample solution and the amount of produced glucose was measured. Digestible dextrin was also analyzed as a control. The glucose amount derived from α-1,6 glucans was lower than from dextrin at all time points, and the glucose production from α-1,6 glucans was inversely proportional to DP. Twenty-four millimoles glucose was produced from dextrin in 2 h, with no significant change observed thereafter. In contrast, 22.8, 20.8, 9.8, and 8.2 mM glucose were produced from DP 3–9, DP 10–30, DP 31–150, and DP 151+, respectively, after 24 h.
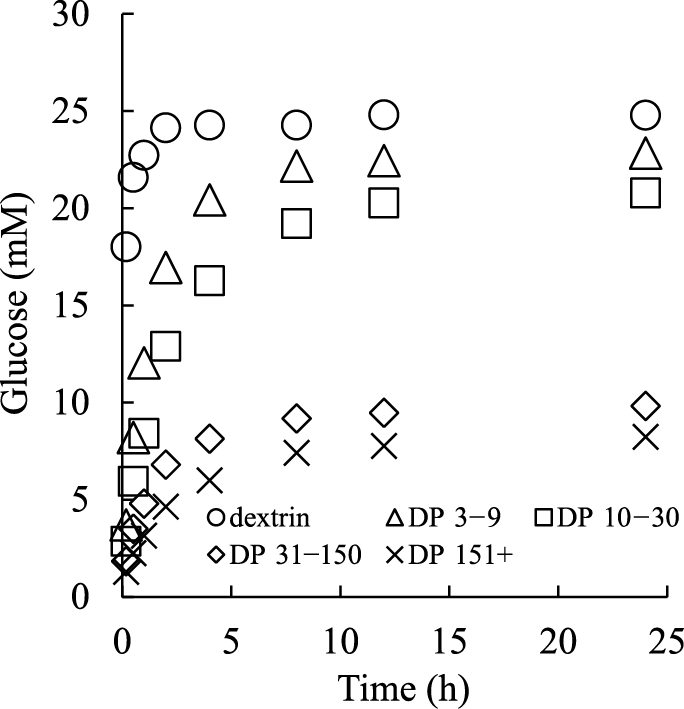
DP 3–9 (△), DP 10–30 (□), DP 31–150 (◇), DP 151+ (×), and dextrin (○).
Postprandial glucose response to α-1,6 glucan from dextran.
We examined the effects of the four types of α-1,6 glucan from dextran on postprandial blood glucose levels in rats. In comparing DP 3–9 to dextrin, no significant difference was found in blood glucose levels at 0–90 min after administration, whereas at 120 min the blood glucose levels in the DP 3–9 group were significantly higher than in the dextrin group (Fig. 2A). In comparing DP 10–30 to dextrin, blood glucose levels 60 min after administration of DP 10–30 were significantly lower than in the dextrin group. (Fig. 2B). Evaluations of the DP 31–150 and DP 151+ groups indicated that blood glucose was significantly lower than dextrin at all time points following administration (Fig. 2C, D). The iCmax values of DP 10–30, DP 31–150, and DP 151+ were significantly lower than those of dextrin (Table 2). The iAUC for DP 3–9, DP 10–30, DP 31–150, and DP 151+ were 99.5, 84.3, 65.4, and 40.1 % relative to dextrin, respectively (Table 2). The iAUC values of DP 31–150 and DP 151+ were significantly lower than dextrin.

Differences in postprandial blood glucose in rats between dextrin (Dex) and DP 3–9 (A), DP 10–30 (B), DP 31–150 (C) or DP 151+ (D). The values are expressed as mean ± SEM for n = 6 (Dex group as a control of DP 31–150), n = 4 (DP 151+ group) or n = 5 rats (others) in each group. *Mean values were significantly different from that of Dex group at same time point (p < 0.05; Student’s t-test).
| Group | iCmax (mg/dL) |
iAUC (mg/dL/min) |
|
|---|---|---|---|
| Dex | 73 ± 9 | 6382 ± 582 | |
| DP 3–9 | 72 ± 5 | 6352 ± 401 | (99.5 %) |
| Dex | 82 ± 9 | 6657 ± 540 | |
| DP 10–30 | 58 ± 4* | 5612 ± 368 | (84.3 %) |
| Dex | 100 ± 5 | 8311 ± 330 | |
| DP 31–150 | 70 ± 3* | 5432 ± 260* | (65.4 %) |
| Dex | 85 ± 5 | 7184 ± 239 | |
| DP 151+ | 40 ± 6* | 2884 ± 388* | (40.1 %) |
The values are expressed as mean ± SEM for n = 6 (Dex group as a control of DP 31–150), n = 4 (DP 151+ group) or n = 5 rats (others) in each group. *Mean value was significantly different from that of the Dex group in the same test (p < 0.05; Student’s t-test). Mean iAUC values (%) relative to the Dex group of each α-1,6 glucan from dextran in the same test are also presented in parentheses.
Preparation of a-1,6 glucan by DDase.
α-1,6 glucan was synthesized by DDase from dextrin. Isoamylase, pullulanase and α-amylase were also used. An aliquot of the product was applied to gel filtration chromatography to remove DP 1–2. The composition and α-1,6 glycosidic bond content of the products is shown in Table 3. The content of DP 1, DP 2, DP 3–9, DP 10–30, DP 31–150, and DP 151+ of the DDase product was 2.4, 3.8, 31.5, 39.8, 9.7, and 12.8 %, respectively. That of the DDase product (–DP 1–2) was 0.0, 0.0, 16.7, 51.1, 11.4, and 20.8 %, respectively. Moreover, the DP 3–9 content decreased with DP 1–2 removal. The content of α-1,6 glycosidic bonds in the above two products was 77.0 and 77.9 %, respectively.
| Composition (%) | α-1,6 bond (%) |
||||||
|---|---|---|---|---|---|---|---|
| Product | DP 1 | DP 2 | DP 3–9 | DP 10–30 | DP 31–150 | DP 151+ | |
| DDase | 2.4 | 3.8 | 31.5 | 39.8 | 9.7 | 12.8 | 77.0 |
| DDase (–DP 1–2) | 0.0 | 0.0 | 16.7 | 51.1 | 11.4 | 20.8 | 77.9 |
In vitro digestibility of DDase products.
The digestibility of DDase product is shown in Fig. 3. Digestibility was analyzed with the same method used for the dextran degradation products. The amount of glucose in DDase products was lower than in dextrin at all time points. The glucose amounts in DDase product (–DP 1–2) was slightly lower than in DDase product. Twenty-four millimoles glucose was produced from dextrin in 2 h, and no significant change was observed thereafter. In contrast, 19.1 and 17.1 mM glucose was produced from DDase product and DDase product (–DP 1–2), respectively, at 24 h.

DDase product (△), DDase product (–DP 1–2) (□) and dextrin (○).
Postprandial glucose of DDase products.
In the in vivo study, the DDase product showed significantly lower blood glucose levels than dextrin at 60 min post-administration (Fig. 4). The DDase product (–DP 1–2) showed a declining trend in blood glucose levels compared with dextrin at 60 min post-administration (p = 0.066). Similarly, the iCmax value of the DDase product was significantly lower than that of dextrin, and the iCmax value of the DDase product (–DP 1–2) showed a declining trend compared to that of dextrin (p = 0.063) (Table 4). The mean iAUC values of the DDase product and DDase product (–DP 1–2) were 89.2 and 86.0 %, respectively, relative to dextrin; however, the difference was not statistically significant (Table 4).

Differences in postprandial blood glucose levels in rats between dextrin (Dex), DDase product (DDase) or DDase product lacking DP 1–2 (DDase (–DP 1–2)). The values are expressed as mean ± SEM for n = 6 rats in each group. *Mean values were significantly different from that of Dex group at same time point (p < 0.05; Dunnett’s test).
| Group | iCmax (mg/dL) |
iAUC (mg/dL/min) |
|
|---|---|---|---|
| Dex | 98 ± 5 | 7339 ± 289 | |
| DDase | 82 ± 5* | 6544 ± 419 | (89.2 %) |
| DDase (-DP 1-2) | 84 ± 3 | 6315 ± 200 | (86.0 %) |
The values are expressed as mean ± SEM for n = 6 rats in each group. *Mean value was significantly different from that of the Dex group (p < 0.05; Dunnett’s test). Mean iAUC values (%) relative to Dex group of each DDase product are also presented in parentheses.
The effect of the DP of α-1,6 glucan on digestibility was investigated in vitro using hydrolyzed dextran. It was shown that α-1,6 glucan with higher DP exhibited an increased resistance to digestion. Increases in blood glucose following administration of α-1,6 glucan with higher DP also tended to be more strongly attenuated. DDase product, which includes various DP fractions of α-1,6 glucan, also attenuated increases in blood glucose. These results suggest that the digestibility of α-1,6 glucan is dependent on its DP.
Lee et al. evaluated the highly branched glucan BEBA, which contains 12.9 % α-1,6 glycosidic bonds at branching structures in an inner main chain composed of α-1,4 glycosidic bonds.8) Shimada et al. evaluated the glucan EHBdex, which contains 8.9 % α-1,6 glycosidic bonds at the non-reducing ends and 5.2 % α-1,6 glycosidic bonds at branching structures in an inner main chain composed of α-1,4 glycosidic bonds, as well as the glucan SHBdex, which contains 2.0 % α-1,6 glycosidic bonds at the non-reducing ends and 13.7 % α-1,6 glycosidic bonds at branching structures in an inner main chain composed of α-1,4 glycosidic bonds.9) The content of α-1,6 glycosidic bonds was increased by enzymatic reactions. All of these glucans contain α-1,4 glycosidic bonds as the main glycosidic bond and α-1,6 glycosidic bonds at similar proportions to each other. In their report, all glucans were less digestible in vitro than the un-treated glucan (the control carbohydrate), and produced different effects on blood glucose levels in vivo. The area under the curve (AUC) of blood glucose levels after administration of BEBA and SHBdex were similar to that of the control carbohydrate, while the AUC of EHBdex was smaller than that of the control carbohydrate. These results indicated that the position of α-1,6 glycosidic bond is more important in determining blood glucose levels than the proportion of α-1,6 glycosidic bonds. Furthermore, α-1,6 glycosidic bonds at non-reducing ends contributed more to reducing blood glucose than α-1,6 glycosidic bonds at branching structures in the inner chain. From these previous studies, we surmised that linear chain glucans consisting almost entirely α-1,6 glycosidic bonds (including the non-reducing end) might attenuate blood glucose increases depending on the DP of the glucan. Notably, dextran is indigestible,11) while isomaltooligosaccharide in the range of DP 2–5 is digestible.12) Our results showed that α-1,6 glucan with moderate DP is digested, in contrast to dextran, and its digestion proceeds more slowly than dextrin.
In this study, α-1,6 glucan with a high proportion of α-1,6 glycosidic bonds and a specific range of DP was prepared by fractionating dextran hydrolysates. Therefore, the effect of DP on digestibility could be evaluated and it was confirmed that the DP of α-1,6 glucan affects digestibility. As shown in Fig. 1, the digestibility of α-1,6 glucan was more attenuated at higher DP and the digestibility of DP 31–150 and DP 150+ was lower than that of the other two samples. These results might be explained as follows. Ingested α-glucans are hydrolyzed by salivary and pancreatic α-amylases, as well as small intestine mucosal two-enzyme complexes of maltase-glucoamylase and sucrase-isomaltase. The α-amylases cannot hydrolyze α-1,6 glycosidic bonds,13) thus α-1,6 glucan is decomposed by mucosal enzymes. Maltase and isomaltase have high α-1,6 glycosidic bond hydrolysis activity compared to the two other mucosal enzymes.14) However, except for glucoamylase, mucosal enzymes decrease their hydrolysis activities with high DP glucan.15) These previous studies are consistent with our results. As expected, α-1,6 glucan with DP 3–9 was largely degraded at 24 h in vitro by small intestine enzymes. DP 10–30 was degraded more slowly than DP 3–9. Proportions of the DP 31–150 and DP 150+ fractions were indigestible at the end of the in vitro reaction. This suggests that small intestine enzymes cannot hydrolyze α-1,6 glucan with high DP. In this study, we found that α-1,6 glucan with a higher DP range tended to be digested more slowly, and α-1,6 glucan with too high a DP was likely indigestible. However, the detailed relationship between DP and digestibility of α-1,6 glucan could not be revealed, because only four fractions were analyzed and two of these fractions, DP 30–150 and DP 150+, consisted of wide DP ranges. Moreover, it was difficult to prepare a fraction with only α-1,6 glycosidic bonds. Therefore, we could not confirm the numerical relationship between DP of pure α-1,6 glucan and digestion rate, or whether the digestion rate changes at a certain DP. To fully understand this phenomenon, it is necessary to evaluate further fractionated products.
Among the dextran degradation products, DP 3–9 showed 92.1 % in vitro digestibility compared to dextrin at 24 h. However, both the iCmax and iAUC of DP 3–9 did not differ from those of dextrin, although the increase in blood glucose seemed to be delayed compared to dextrin. This indicated that DP 3–9 was as rapidly digested as dextrin in vivo. Since the gastrointestinal tract of rats exhibits more digestive activity than the in vitro environment in this study, it is thought that DP 3–9 was digested to almost the same level as dextrin. Previous studies have also reported that in vitro evaluations reflect the digestive status of living organisms to some extent, but do not entirely correspond to the in vivo environment.16) This is likely attributable to differences in the amount of enzyme involved in digestion and the specific reactions occurring only in vivo, such as gastrointestinal peristalsis. On the other hand, α-1,6 glucan with DP > 10 exhibited low digestibility in vitro and good suppression of blood glucose increase in vivo, with these effects becoming stronger as DP increases. The suppression of blood glucose levels may results from slow digestibility or partial indigestibility of α-1,6 glucan. In contrast, the equivalent iAUC to the known digestible carbohydrate may result from high digestibility. Therefore, DP 10–30 is expected to be a slowly digestible glucan that may undergo complete digestion, because it was digested less than DP 3–9 in vitro, and it produced significantly lower maximum blood glucose levels than dextrin with no significant difference in iAUC in vivo. We assume that DP 31–150 and DP 151+ contain indigestible glucan, since they exhibit low digestibility in vitro and in vivo. However, one limitation of this study is that the digestibility of these glucans could not be completely confirmed from these results alone. Thus, evaluation of bioavailability is needed to determine whether α-1,6 glucan with DP > 10 is slowly digestible or indigestible in vivo. Methods are available for evaluating the bioavailability of α-1,6 glucan, including measurement of exhaled hydrogen gas to determine whether the glucan reaches the colon to be fermented, measurement of the amount of glucan excreted from feces and evaluation of the emission of exhaled CO2 from labeled glucan.12)17)18)
In the preparation of the DDase product, the reaction was controlled to primarily contain DP 10–30. This was chosen because α-1,6 glucan of DP 10–30 was expected to produce the greatest degree of attenuation of postprandial blood glucose levels, and be completely digested (according to the results of dextran degradation products). The DDase product consisted of both α-1,4 glycosidic bonds at the reducing ends and α-1,6 glycosidic bonds at non-reducing ends, and mainly included the DP 10–30 fraction and a small amount of DP 1–2, as shown in Table 3. The DP 1–2 fraction, in which glucose, maltose and isomaltose are included, is easily digested and absorbed. Therefore, the DDase product contains easily digestible and slowly digestible glucan fractions and may also contain an indigestible glucan fraction. While it was possible to fractionate the DDase product in a manner similar to the dextran degradation products, the evaluation of the DDase product was performed without fractionation to avoid high costs in industrial use. However, an additional sample was prepared that excluded only DP 1–2, in order to estimate their influence. To form α-1,6 glycosidic bonds effectively, isoamylase, pullulanase and α-amylase were also used in the DDase reaction. Importantly, the DDase product contained 23.0 % α-1,4 glycosidic bonds. Therefore, the DDase product is presumed to be more digestible than the dextran degradation product at equivalent DP. As shown in Fig. 3, dextrin produced 24.0 mM of glucose in 2 h. On the other hand, the DDase product and DDase product (–DP 1–2) showed low digestibility in vitro and produced 19.1 and 17.1 mM of glucose in 24 h, respectively, which is comparable to hydrolyzed dextran of DP 10–30 (20.8 mM). The presence of DP 1–2 affected the amount of glucose produced in the early stages of the reaction. In vivo, the DDase product showed a significantly lower iCmax value than dextrin. The DDase product (–DP 1–2) also showed a declining trend in iCmax value compared to dextrin. The mean iAUC values of DDase product and DDase product (–DP 1–2) were 10.8 and 14.0 % lower than that of dextrin, respectively; however, the difference was not statistically significant. The properties of DDase products, such as digestibility in vitro and increases in postprandial blood glucose levels in vivo, seem to be similar to those of hydrolyzed dextran of DP 10–30. Taken together, the digestibility of DDase product and DDase product (–DP 1–2) was comparable to that of hydrolyzed dextran of DP 10–30. Although the exclusion of DP 1–2 delayed the digestion rate in vitro, its effect on blood glucose in vivo was minor. On the other hand, the DDase product contains 23–32 % of DP ≥ 31 fractions, which appeared to be indigestible in the in vitro evaluation of dextran degradation products. It is possible that these fractions imparted indigestibility to the DDase product. However, it remains unclear whether the DDase product may be slowly digestible or contains indigestible fractions, as well as which DP is completely digested and absorbed in the small intestine.
In this study, we found that the DP of α-1,6 glucan is related to its digestibility and blood glucose increase. In addition, α-1,6 glucan with DP 10–30 is likely to be a slowly digestible carbohydrate because this glucan produced significantly lower maximum blood glucose levels than dextrin with no significant difference in iAUC in vivo. It was also confirmed that this α-1,6 glucan could be synthesized by DDase as well as the degradation of dextran. Furthermore, it was revealed that DDase product with a wide range of DP showed slow digestibility and good attenuation of blood glucose increases. The DDase product can be synthesized from dextrin, which is an inexpensive carbohydrate material. Therefore, the DDase method can be applied to industrial production of α-1,6 glucan. Further research into the evaluation of bioavailability and blood glucose responses in humans is required in order to utilize the DDase product as a slowly digestible carbohydrate. Ultimately, this glucan may replace dextrin in liquid diets for elderly diabetic patients to provide sufficient energy without a rapid increase in blood glucose after ingestion.
Kenta Aizawa, Hiroki Takagi and Masayasu Takada are employees of Nihon Shokuhin Kako Co., Ltd. Eri Kokubo is employee of Morinaga Milk Industry Co., Ltd.