2022 Volume 69 Issue 4 Pages 73-81
2022 Volume 69 Issue 4 Pages 73-81
This study aimed to characterize the interactions between cereal flour (rice, wheat, and barley) and “nata puree” (NP), a disintegrated bacterial cellulose (BC) in the presence of a water-soluble polysaccharide, with powder-dispersion activity. Pasting properties of cereal flour with additives were analyzed using a Rapid Visco Analyzer, and disintegrated BC in water (BCW), three water-soluble polysaccharides: (1,3)(1,4)-β-glucan, tamarind seed gum, and birchwood xylan, and the corresponding NPs were used as additives. For rice flour, additional BCW or NPs increased the initial and the peak viscosity. The addition of water-soluble polysaccharides produced the opposite trend: viscosity increased from the peak time to the end of measurements. For wheat flour, the addition of BCW or NP delayed the peak time and increased peak viscosity; the increase was maintained till the end of measurements. For barley flour, the additional BCW or NP caused a higher gelatinization rate and increased viscosity at the starch-retrogradation stage. Next, static gelatinization of a rice flour suspension in NP was successfully accomplished before placing it in a vessel; NP concentration in the gel significantly affected the firmness. Thus, the dynamic and unique interactions between various cereal flours and cell-wall polysaccharides in NPs can increase the flours' potential; static gelatinization of cereal flour with NP could expand flours' application range in both current and next-generation cooking.
BC, bacterial cellulose; NP, nata puree; BG, (1,3)(1,4)-β-glucan; TG, tamarind seed gum; BX, birchwood xylan; OD, optical density; RVA, Rapid Visco Analyzer; NPXX, NP sample made with polysaccharide “XX”; BCW, disintegrated BC sample with water; RW, a suspension of rice flour in water; RNP25, a suspension of rice flour in 25 % (w/v) NPTG (the final concentration); RNP50, a suspension of rice flour in 50 % (w/v) NPTG (the final concentration).
Cereal, a staple food for people worldwide, is an important energy- and carbohydrate source. Depending on cooking culture, the grain rigidity, and so on, it can be stored and cooked either as grain- or flour. Cereal flour, a homogeneous powder, allows more reproducible cooking over the corresponding grains, especially when measured, mixed with other components, heated, and gelatinized. The high performance of flour in cooking has been adapted to conventional foods such as bread, pasta, noodles, and cookies. Recently, flour has been regarded as an excellent ingredient for 3D food printing, a next-generation food design and preparation system for three-dimensional food formulation using extrusion, inkjet printing, binder jetting, etc.1)2) In addition to the high performance of flour for reproducible preparation, the large starch content in cereal flour doughs could help maintain the stability of the prepared structures.3)4)5)
Meanwhile, flour suspensions at lower concentrations than for doughs are used in conventional cooking as a batter, a flour suspension to cover foods such as fish or chicken before frying. Additionally, the term batter can be applied to dilute flour suspensions for pancakes and rice paper. In practical cooking or in laboratory experiments, flour is mechanically suspended in a batter just before heat gelatinization.6) The use of batter in 3D food printing is expected to extend the range of texture expressions by starch-gel formation; however, flour has a high specific gravity and a low dispersibility in the batter; flour precipitation before its gelatinization could result in an inhomogeneous gel and unstable printing.
Therefore, a new method for flour dispersion in batter is required for starch-gel activity in 3D food design. In this study, we focused on “nata puree (NP),” a disintegrated bacterial cellulose (BC) in the presence of a water-soluble polysaccharide, as a new additive for stabilizing flour dispersion in batter.7) In that report, NP was shown to disperse both potato powder and potato starch, and NP addition caused a dramatic change in the pasting profile of a suspension of potato powder, maintained throughout measurement time and with an exceptionally larger effect at peak viscosity. The profile suggests that the cell wall components in potato powder could interact with cellulose and/or a water-soluble polysaccharide [(1,3)(1,4)-β-glucan (BG)] in the NP.
Herein, we characterized the interaction between NP and rice, wheat, and barley cereal flour by analyzing the pasting properties. We already have data of the corresponding interaction of dicot samples such as potato powders and potato starch, whereas the cell wall structure of monocots is quite different from that of dicots; BG, arabinoxylan, glucuronoxylan, xyloglucan, etc., are observed as the main hemicellulosic components in monocots.8) In addition, the hemicellulosic components in cereal grains are distributed not only in the aleurone layer but also the endosperm, suggesting that dynamic interactions between NP and cereal flour could be expected during starch gelatinization and retrogradation. We also demonstrated NP's powder-dispersion activity through static gelatinization of a rice flour batter for stable gel formation and discuss the impact of this process on next generation cooking.
Materials. Dice-shaped nata de coco blocks (Morinaga nata de coco plain) were purchased from Morinaga Milk Industry Co., Ltd., Tokyo, Japan, and de-syrupped for purification of bacterial cellulose (BC) as described in our previous report.7) BG (P-BGH, high viscosity, Megazyme Ltd., Bray, Ireland), lichenase (endo-1,3:1,4-β-D-glucanase: E-LICHN, Megazyme; 273 U/mg protein), and xylanase (endo-1,4-β-xylanase: E-XYNACJ, Megazyme; 38 U/mg protein) were purchased from Biocon (Japan) Ltd., Nagoya, Japan. Tamarind seed gum (Tamarind gum, TG) and birchwood xylan (BX) were purchased from Tokyo Chemical Industry Co., Ltd., Tokyo, Japan and Sigma-Aldrich Japan, Tokyo, Japan, respectively.
Rice flour was prepared by air-flow jet-milling of Nipponbare white rice. Wheat flour (soft wheat flour, Hakurikiko) was purchased from Nisshin Flour Milling Inc., Kawasaki, Japan. Barley grain (Hakubaku Ltd., Chuo, Japan) was milled to pass through a 1-mm mesh sieve using a flour mill (SRG 05C, SATAKE Corporation, Higashi-Hiroshima, Japan); the recovered powder was heated at 70 °C for 18 h for enzyme inactivation. Other chemicals were of reagent grade.
Preparation of NP samples. BC disintegration in water only and in the presence of BG, TG, and BX was performed for preparation of the corresponding samples called BCW, NPBG, NPTG, and NPBX, respectively, using a modified version of our previously described protocol:7) 20 g of de-syrupped BC (corresponding to 138 mg dry BC) and 27 mL of a solution of 0.35 % (w/v) water-soluble polysaccharide (0.27 % (w/v) final concentration for BC, TG, and BX) were mixed for disintegration at 25,000 rpm for 30 s using a blender (Foodmill TML180, Tostem, Tokyo, Japan); disintegration was repeated three more times. For BCW preparation, 27 mL of distilled water, instead of a solution of a water-soluble polysaccharide, was mixed with de-syrupped BC blocks for disintegration following the same protocol as above. The stock solutions of water-soluble polysaccharides for a rheological analysis were prepared by BC, TG, or BX solubilization in distilled water at a final concentration of 0.1 % (w/v). The samples were kept at 4 ºC until use.
Particle size distribution analysis with a testing sieve. BCW, NPBG, NPTG, and NPBX were diluted 4.7 times with distilled water (w/v); then, the diluted samples were gently stirred for 3 min and degassed for 5 min to purge bubbles. The optical density (OD) at 600 nm (OD600 nm) of each suspension was measured using a spectrophotometer (SpectraMax Plus 394, Molecular Device Japan, Tokyo, Japan) in a 1-mL cuvette with a path length of 10 mm. The measurements were repeated four times, and the average value was calculated. Then, the suspension (40 mL) was poured onto a testing sieve (aperture: 0.3 mm; diameter: 75 mm, Tokyo Screen, Co., Ltd, Tokyo, Japan). The OD600 nm of the filtrate was measured as described above. All these procedures were performed twice, and the average value of the two experiments and standard deviation were calculated for each suspension.
Rheological analysis. The pasting properties were analyzed using a Rapid Visco Analyzer (RVA) (model RVA4800, Perten Instruments Pty. Ltd., NSW, Australia). The powder suspension was prepared by adding 3.0 g of the flour (rice, wheat, or barley) to a canister containing 25.0 mL of water, a solution of water-soluble polysaccharide [solid content of 0.05 % (w/v)], a diluted BCW [solid content of 0.0725 % (w/v)], or a diluted NP [total solid content of 0.1225 % (w/v)] and mixing them thoroughly. The viscosity profile was recorded using the Thermocline software for Windows ver. 3.17 under the following conditions: each suspension was kept at 50 °C for 1 min, heated up to 95 °C in 3.7 min, held for 2.5 min at 95 °C, cooled to 50 °C in 3.8 min, and kept for 2 min at 50 °C. The paddle was rotated at 960 rpm for the first 10 s after starting RVA analysis, and then constantly rotated at 160 rpm. Parameters such as pasting temperature, peak viscosity, time at peak viscosity, holding strength, and final viscosity were recorded. For the analysis of the effects of hemicellulolytic enzymes on the viscosity profile, additional procedures were adopted at the last part of the condition shown above: the paddle rotation was stopped for 1 min at 50 °C for the addition of the enzyme, and the paddle re-rotated at 160 rpm for 20 min at 50 °C. For the enzyme sample, lichenase (10 U) and/or xylanase (5 U) was added to the canister in either an active- or a heat-denatured form.
The effects of NP addition on the physical properties of rice paste samples were investigated using a rheometer (CR-500DX, Sun Scientific Co., Ltd., Tokyo, Japan). Rice flour was mixed with distilled water or a diluted NPTG solution with distilled water; the final NPTG concentration was 25 % (w/v) or 50 % (w/v) of the original NPTG concentration. The final rice flour concentration was fixed at 7.5 % (w/v), and the three samples at a final NPTG concentration of 0, 25, and 50 % were called as RW, RNP25, and RNP50, respectively. The samples were placed in hot water at 95 °C for 30 min; RW was manually shaken for maintain flour dispersed during gelatinization, whereas static gelatinization without manual shaking was performed for RNP25 and RNP50. A plastic dish (diameter: 60 mm, height: 10 mm) was filled with each sample just after gelatinization, and the sample was settled for cooling to room temperature for rheological analysis. The analytical parameters were obtained according to a previous report:9) a cylindrical plastic plunger (diameter: 10 mm, height: 20 mm) was lowered until contact with the top of the sample, further penetrating to a depth of 5 mm at 1 mm/s. Then, the plunger was lifted at the same speed, and the force vs. time was plotted. The firmness peak and the adhesiveness area were calculated.10)
Statistics. Tukey's test was performed with the software KyPlot 6.0 (KyensLab Incorporated, Tokyo, Japan). Differences were considered significant at p < 0.05 (*) or p < 0.01 (**).
Preparation of NPs.
In our previous report, NP preparation was developed based on the fact that a BC gel can be more efficiently disintegrated in the presence of BG than in its absence.7) Herein, we adopted a smaller blender and lower BC concentration than previously. We also used two kinds of water-soluble polysaccharides: TG and BX in addition to BG for NP preparation. Figure 1 shows that NPBG, NPTG, and NPBX were prepared in a well-disintegrated form, as confirmed by passing through a stainless mesh with a pore diameter of 0.3 mm. Eighty percent of the particles in the BCW were trapped on the mesh, suggesting insufficient disintegration.
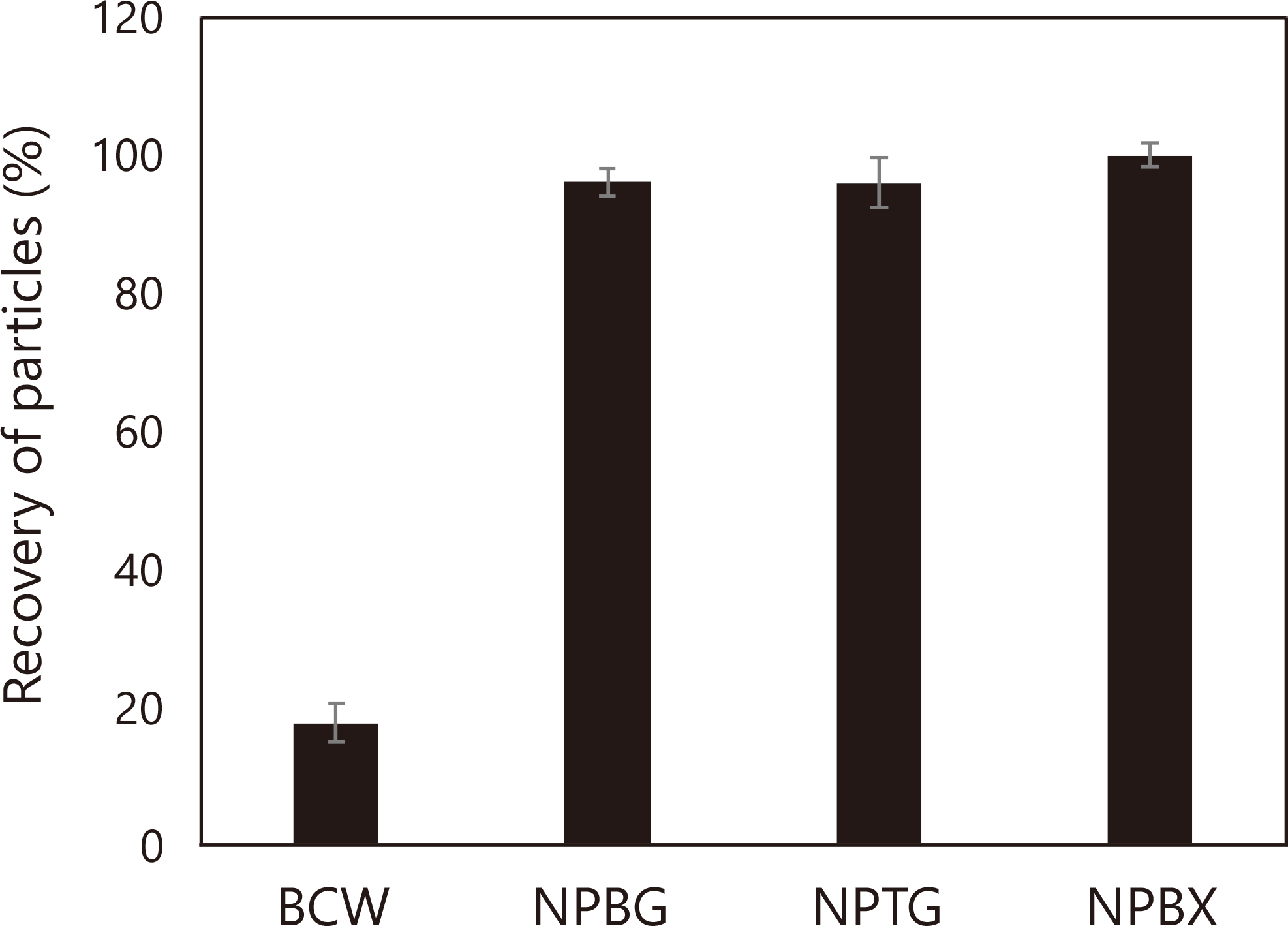
Turbidity was measured as OD600 nm; the ratio of turbidity after passing through a 0.3 mm filter is indicated. Results are expressed as means ± SD (n=2).
BG, xyloglucan (the main component of TG), and arabinoxylan are main hemicelluloses in the cell walls of monocotyledons.8) Their specific interactions with cellulose have been investigated using BC11) or microcrystalline cellulose12) as model cellulose. The preparation of a series of NP samples using various hemicelluloses could provide unique bio-functional- and/or physicochemical properties of both cellulose and individual hemicelluloses. In addition, fine structures of the hemicelluloses significantly affect the interaction with cellulose, as proven with the side chains of xyloglucan13) and xylan.14) In this study, instead of arabinoxylan, we used BX, a glucuronoxylan, based on its excellent BC-disintegrating activity and its existence in rice-grains.8)
Pasting properties of rice flour in the presence of additives.
The RVA analysis of cereal flour unveils functional properties such as gelatinization, pasting, and retrogradation, which could be determinants of the suitability of each flour to a certain use.15)16) Figure 2A shows the RVA profile of rice flour with and without BCW: the addition of BCW slightly increased the initial viscosity, without affecting other important parameters like peak viscosity, time at peak viscosity, or holding strength. The final viscosity slightly decreased with BCW addition. A similar trend was observed in samples with NPBG, NPTG, and NPBX, whereas the difference in initial viscosity was constant till peak viscosity with NPBG, NPTG, and NPBX (Figs. 2B-D). When the corresponding water-soluble polysaccharides were used alone, the trend differed from that with BCW and NPs; the addition did not increase viscosity at the gelatinization stage, but it slightly increased the peak viscosity, holding strength, and the final viscosity (Figs. 2B-D). This trend with the polysaccharides was less obvious with the corresponding NPs, probably because part of the polysaccharides could be associated with BC.

A: experiments for BCW, B: experiments for NPBG and BG, C: experiments for NPTG and TG, D: experiments for NPBX and BX. + BCW: a flour suspension with BCW, Ctrl: a flour suspension in water, Temp: temperature, + NPXX: a flour suspension with the corresponding NP made from polysaccharide “XX”, + XX: a flour suspension with polysaccharide “XX.”
In our previous report, a large increase in viscosity before gelatinization was observed, suggesting that the cell wall components from potato powder could interact with NPBG before gelatinization.7) Such a phenomenon was not observed with rice flour and additives, suggesting that exposed and/or leached components in the flour could have minimum effects on the viscosity increase before gelatinization. At the initial gelatinization stage, newly exposed- and/or leached components could interact with the cellulose in BCW and NPs, because the corresponding hemicelluloses alone as the additives (i.e., BG, TG, and BX) did not increase the viscosity. The lower effect of BCW on viscosity than NPs may be attributed either to different particle sizes (Fig. 1) or a synergetic effect of the two components in NPs. The loss of the effect at the peak viscosity in samples with BCW and NPs might be caused by disruption of the starch structure, which could also break the network with flour and the cellulosic additives.
Because of data limitations, the difference in viscosity at the holding strength to the final viscosity caused by addition the water-soluble polysaccharides cannot be explained. However, the effect of BG on the rheological properties of rice flour dough suggests an interaction between BG and rice flour.17) It is possible that a new cellulosic surface is exposed from flour fragments after starch disruption, which could interact with the polysaccharides to increase viscosity, as high interactivity with cellulose is the common property of all three polysaccharides.11) Furthermore, the cell wall from rice grains contains 5-10 times more α-cellulose than that from wheat,18) which could support our speculation.
Pasting properties of wheat flour in the presence of additives.
BCW had three effects on the pasting properties of wheat flour, an increase in the initial viscosity, a delay in peak time, and an increase in viscosity from peak time to the end of measurements (Fig. 3A). The RVA profile of wheat flour exhibited a slow gelatinization phase for about 2 min at the beginning19) (Fig. 3A). BCW was shown to affect the interaction at this slow gelatinization phase, which could reflect the initial structural changes of wheat flour. In Figure 3A, a rapid increase of viscosity by starch gelatinization was observed after the slow gelatinization phase; the addition of BCW delayed gelatinization by 1 min, which was reflected in a delay in peak time. BCW did not affect the maximum speed of rapid gelatinization but increased peak viscosity. The difference of viscosity caused by the BCW addition was constant from peak time to the end of measurements, suggesting that this difference is independent of the structural changes of starch during disruption and retrogradation.

A: experiments for BCW, B: experiments for NPBG and BG, C: experiments for NPTG and TG, D: experiments for NPBX and BX. + BCW: a flour suspension with BCW, Ctrl: a flour suspension in water, Temp: temperature, + NPXX: a flour suspension with the corresponding NP made from polysaccharide “XX”, + XX: a flour suspension with polysaccharide “XX.”
Next, the effects of NPs and the corresponding polysaccharides on the properties were evaluated (Figs. 3B-D). NPs showed the same effect as BCW; peak viscosity with NPBX looked like that with BCW, whereas in samples with NPBG and NPTG it was larger than with BCW. When the corresponding water-soluble polysaccharides were used as additives, the effects were limited (Figs. 3B-D). The larger effect of NPBG and NPTG than BCW may be attributed to the presence of BG or TG on the NPs' surface, which may enhance the interaction between components in wheat flour and the NPs.
The addition of BCW increased the final viscosity; the effects of two hemicelluloses, xylan and BG, in wheat flour were investigated by a prolonged profile analysis after addition of an enzyme. As shown in Fig. 4A, a dramatic decrease in viscosity was observed in a control sample (without BCW) with xylanase, whereas lichenase had no effects on viscosity. Enzyme addition caused a similar trend in a sample with BCW (Fig. 4B). The effect of xylanase was larger in the sample with BCW than without it, suggesting that an interaction between the cellulose in BCW and xylan in the flour could contribute to the higher final viscosity than in the control. In contrast, when rice flour was used for this prolonged measurement, neither lichenase nor xylanase decreased viscosity (Fig. S1; see J. Appl. Glycosci. Web site).

A: experiments for a flour suspension in water, B: experiments for a flour suspension with BCW. Ctrl: a flour suspension in water, Ctrl + lichenase: Ctrl plus lichenase, Ctrl + xylanase: Ctrl plus xylanase, Temp: temperature, + BCW: a flour suspension with BCW, + BCW + lichenase: + BCW plus lichenase, + BCW + xylanase: + BCW plus xylanase.
Heat-inactivated enzymes had no effects on the profile (data not shown).
Pasting properties of barley flour in the presence of additives.
The pasting properties of barley flour in the presence of BCW are shown in Fig. 5A. No clear interaction of BCW with the flour was observed before pasting time. The increase in viscosity at the gelatinization stage was higher with BCW, which resulted in larger peak viscosity. The faster gelatinization might be attributed to a synergetic effect of starch gelatinization and an interaction between BCW and components in the flour. The peak viscosity was reached earlier with BCW than without, suggesting the faster starch disruption. Then, the viscosity rapidly decreased by starch disruption, and the holding strength of the sample with BCW became equivalent to that without it. This result suggests that non-disrupted starch in the flour is a prerequisite for increased viscosity at the gelatinization stage.

A: experiments for BCW, B: experiments for NPBG and BG, C: experiments for NPTG and TG, D: experiments for NPBX and BX. + BCW: a flour suspension with BCW, Ctrl: a flour suspension in water, Temp: temperature, + NPXX: a flour suspension with the corresponding NP made from polysaccharide “XX”, + XX: a flour suspension with polysaccharide “XX.”
We observed another unique effect of BCW at the late measurement stage: the increase of viscosity restarted just after cooling (Fig. 5A). The difference in viscosity between the two samples appears to be constant when the temperature decreased < 70 ºC at the retrogradation stage, indicating that viscosity increases only at the initial stage of starch retrogradation.
NPs exhibited similar effects on barley flour to BCW, whereas a larger baseline shift from the beginning of the measurement was observed (Figs. 5B-D), which may be attributed to differences in particle size after disintegration (Fig. 1). The peak viscosities of samples with NPBG and NPTG were slightly larger than with BCW, whereas the corresponding value of the sample with NPBX was equivalent to that with BCW. In samples with the corresponding water-soluble polysaccharides, the trend is similar to the control without additives, except that a slight increase in peak viscosity compared with that without any additives, as observed in the sample with NPBG.
Figure 6 shows the same set of experiments as in Fig. 4, using barley flour instead of wheat flour. Lichenase reduced the final viscosity by 50 %, whereas xylanase slightly increased the viscosity during prolonged paddle rotation (Fig. 6A); the same trend was observed in the sample with BCW (Fig. 6B). Unlike with xylanase in wheat flour samples, the effect of lichenase was equivalent between the sample with and without BCW, indicating no lichenase-susceptible interactions between the cellulose in BCW and BG.

A: experiments for a flour suspension in water, B: experiments for a flour suspension with BCW. Ctrl: a flour suspension in water, Ctrl + lichenase: Ctrl plus lichenase, Ctrl + xylanase: Ctrl plus xylanase, Temp: temperature, + BCW: a flour suspension with BCW, + BCW + lichenase: + BCW plus lichenase, + BCW + xylanase: + BCW plus xylanase.
Heat-inactivated enzymes had no effects on the profile (data not shown).
Dietary fibers are desirable not only for its health benefits but also for creating unique textures alone and/or by their interactions with other food materials. In this study, we demonstrated that the pasting properties of rice-, wheat-, and barley flour are differently affected by dietary fibers (i.e., cellulose and hemicellulose). When a conventionally used flour is substituted by another one; for example, rice flour is used instead of wheat flour for making gluten-free bread, it is important to understand the interactions between the flour and dietary fibers in other food materials and/or food additives. The differences of cultivars, polishing degree, and remaining enzymes in the flour could also affect the interactions as well as the fine structures of BCW, NP, and water-soluble polysaccharides, which should expand the range of application of individual cereal flour.
Static gelatinization of a suspension of rice flour with NPTG.
NP has flour-dispersing activity (Fig. S2; see J. Appl. Glycosci. Web site), and it was applied to a batter for stable dispersion and static gelatinization of rice flour at a low concentration (7.5 % (w/v)). The suspension of rice flour without NPTG was unable to homogeneously gelatinize unless agitated for a manual flour dispersion (data not shown). However, the corresponding samples with NPTG successfully gelatinized without agitation. The gelatinized samples were transferred to a vessel and cooled to form gels for investigation of their rheological properties (Fig. 7). The static gelatinization of a batter with NPTG produced a homogeneous gel; the firmness peak (Fig. 7A) and the adhesive area (Fig. 7B) were equivalent to or larger than those of the sample without NPTG. NPTG addition significantly increased firmness, in accordance with our previous report using a potato powder paste.7) We also succeeded in static gelatinization in a continuous, automated gel production from a batter of cereal flour and NP; rice flour gel was successfully formed through a simple line with two instruments: a pump for extruding a suspension of cereal flour and an on-line heating device (Fig. S3; see J. Appl. Glycosci. Web site).

A and B indicate sample firmness at 5-mm depth and sample adhesiveness, respectively. RW, RNP25, and RNP50 represent paste samples at a final NPTG concentration of 0, 25, and 50 %, respectively. Two sets of dishes were used for each rice paste. The measurements were performed five times per dish by changing the press point on the surface. Then, the average value excluding both the maximum and minimum values as outliers was calculated; the average ± standard deviation of two plates is shown. Statistical analysis was performed using Tukey’s test; p < 0.05 was considered statistically significant (*p < 0.05, **p < 0.01, n.s.; no significance).
Static flour gelatinization results in formation of a starch network, whereas high-speed shearing of rice gel modifies its rheological properties by reconstructing the gel network, suggesting that the rheological properties of statically gelatinized rice flour could be further modified by mechanical shearing after gelatinization.20) Another way of controlling gel properties would be the addition of gel-forming ingredients such as agar and gelatin; NP might also disperse powdered ingredients, and the static gelatinization step for flour would also gelatinize said powders, as shown in a suspension with curdlan powder.7) For reproduction of various foods by automatic cooking, a great deal of effort would be required to automate various conventional cooking processes. On the other hand, it is expected that we can develop new, unique processes suitable for automatic cooking such as static gelatinization with the aid of NPs.
In conclusion, we analyzed the effects of BCW, NPs and the corresponding water-soluble polysaccharides on the gelatinization and aging behaviors of three flour samples; unique interactions were found for each flour, suggesting specific contributions of flour components. In addition, static gelatinization was successfully achieved by dispersing rice flour in NP, which could promote the use of batter in current- and next-generation cooking. While the importance of food additives such as hydrocolloids and carbohydrates for improving the quality of 3D/4D printed natural food gels has been emphasized,21) the interactions between those additives and natural food materials have not been well characterized. The present findings demonstrate that starch food materials could interact with cellulose and hemicellulose in NPs; a crucial information for controlling properties of food products as well as developing novel food products.
The authors declare no conflict of interests.
This work was supported by Cabinet Office, Government of Japan, Moonshot R&D Program for Agriculture, Forestry and Fisheries (funding agency: Bio-oriented Technology Research Advancement Institution), Grant Number JPJ009237. We are grateful to Ms. Hiromi Nakayama, Ms. Kazuyo Sato, and Ms. Hitomi Yamada for their excellent technical assistance. The authors would like to thank Enago (www.enago.jp) for the English language review.