Diploid wheat Triticum monococcum is considered the progenitor of hexaploid common wheat and tetraploid durum wheat [1, 2, 3]. The application of flour is determined by the amount and composition of its storage proteins and starch, which constitute 10-18 % and 60-75 % of dry seed weight, respectively [4].
Wheat storage proteins comprise several fractions: water-soluble albumins, dilute sodium chloride-soluble globulins, alcohol-soluble gliadins, and dilute acid- or alkaline-soluble glutenins. Among these, gliadins and glutenins, accounting for approximately 75 % of total storage proteins [4], are crucial for gluten formation and essential in bread baking. Gliadins are further classified into α-, γ-, and ω-gliadin, while glutenins are categorized into high and low molecular-weight types [5]. Wheat storage proteins can trigger wheat allergy and celiac disease, with ω-5 gliadin being the most prevalent cause in anaphylaxis [5, 6].
Wheat starch is primarily composed of two types of glucose polymers: linear amylose and branched amylopectin. In the wheat endosperm, amylose synthesis is almost entirely driven by granule-bound starch synthase I (GBSSI), encoded by the Waxy gene [7]. Typical wheat starch contains 20-30 % amylose and 70-80 % amylopectin [8]. The ratio of amylose to amylopectin, along with variations in the frequency and length of amylopectin branches, significantly influence the texture and digestibility of wheat products [9].
The first amylose-free waxy mutant of common hexaploid wheat was reported by Nakamura et al. in 1995 [10]. A key difference between waxy common wheat and other waxy crops is the presence of storage proteins in wheat that enable gluten formation. Gluten reduces adhesiveness and making the dough easier to handle. This, however, does not compromise the soft and chewy texture characteristic of waxy starches. Consequently, incorporating hexaploid waxy wheat flour into bread and confectionery products can enhance their texture [11, 12, 13].
This study employed a previously isolated diploid waxy mutant of T. monococcum, MO3M, which derived from ethyl methanesulfonate-treated mutant populations of var. flavescens, MO3 (Monococcum cultivar 3 or KU105) [14, 15]. MO3M harbors a guanine-to-adenine substitution at nucleotide position 1614. This substitution within exon 6 introduces a stop codon, resulting in the absence of the GBSSI protein [15]. Thus, MO3M is amylose-free and possesses an amylopectin structure similar to its wild-type counterpart, MO3 [14]. This study aimed to investigate the impact of this waxy diploid wheat on food processing applications. Therefore, the nutritional composition, including starch, protein, lipid and fiber contents, were determined. In addition, storage protein composition, gluten content, allergen content, and rheological property of MO3M were compared to its wild-type and a range of hexaploid wheat varieties, including bread, cake, and amylose-free wheats flours.
Both diploid wheat lines, MO3M and MO3, were cultivated in an experimental field at Fukui Prefectural University under natural conditions. Absence of GBSSI in diploid and hexaploid waxy wheat (MO3M and Mochihime, respectively) used in this study were confirmed by western blotting (Fig. S1; see J. Appl. Glycosci. Web site).
Consistent with a previous study, the upper third of the MO3M ear exhibited sterility [14]. Seed weight analysis was performed using ten grains from each line. MO3M seeds exhibited a significantly lower average weight (18.3 ± 1.3 mg) compared to MO3 (27.8 ± 0.7 mg), representing only 66 % of MO3. This finding aligns with previous reports of reduced seed weight in hexaploid waxy wheat mutants [10]. Furthermore, a strong correlation between GBSS activity, starch content, and thousand seed weight has been reported [16].
To investigate changes in nutritional composition, the starch, protein, lipid, and fiber contents of the flours were analyzed. Wheat flour for MO3M and MO3 was prepared from polished wheat kernels using the roller milling method with an SN-CK mill (KOKKO CO.,LTD., Suwa, Japan). Flours from hexaploid waxy wheat (“Mochihime,” Heiwa Flour Milling Co., Osaka, Japan [17]), cake flour (“Flour,” Nisshin STC Flour Milling Co., Ltd., Thailand), and bread flour (“Camellia,” Nisshin STC Flour Milling Co., Ltd.) were commercially sourced from local grocery stores.
Starch content in flour was determined. Briefly, 10 mg of flour was washed with 90 % methanol to remove free sugars. The washed flour was then dried and resuspended in 900 μL of water. The flour suspension was boiled for 1 h, diluted with water to a final volume of 1.5 mL This gelatinized and diluted sample (50 μL) was then incubated with glucoamylase (Megazyme International Ireland, Bray, Ireland) at 60 °C for 1 h to convert the starch into glucose. Glucose content was quantified using the GOPOD format (Megazyme), according to the manufacturer's instructions. The moisture content of the flour samples used for starch analysis was determined using a moisture analyzer (MA150, Sartorius AG. Germany). Starch content was then calculated based on a dry weight basis assuming 14 % moisture content (Table 1). The results revealed that the starch content of MO3M was 65.6 g/100 g, similar to that of the wild-type MO3 (67.5 g/100 g). Bread, cake, and Mochihime flours exhibited starch contents of 64.9 g/100 g, 63.9 g/100 g, and 71.2 g/100 g, respectively.
Table 1. Protein, gluten, total dietary fiber, and allergen content in the wheat lines used in this study.
| Line | Genotype | Starch content (g/100 g) | Protein content (g/100 g) | Lipid content (g/100 g) | Total dietary fiber (g/100 g) | Gluten content (%) | Allergen content (mg/g) |
| MO3 | WT, T. monococcum | 67.5±0.2 | 8.2 | 1.6 | 6.1 | Trace | 56.2±7.5 |
| MO3M | waxy; T. monococcum | 65.6±1.2 | 16.2 | 2.1 | 9.5 | 43.3 ± 4.3 | 96.9±16.3 |
| Cake flour | WT; T. aestivum (Low gluten) | 63.9±2.3 | (8.3) | (1.5) | (2.5) | 28.2 ± 1.1 | 64.4±2.8 |
| Bread flour | WT; T. aestivum (High gluten) | 64.9±3.5 | (11.8) | (1.5) | (2.7) | 43.1 ± 4.0 | 108.7±22.8 |
| Mochihime | waxy; T. aestivum | 71.2±1.8 | (9.5) | (1.4) | (2.7) | 41.2 ± 5.8 | 116.4±18.8 |
The values in the brackets are for reference use, and those of cake and bread flours are from https://fooddb.mext.go.jp/ and Mochihime is from Fujita, 2022 [19]. Wild-type, WT; Triticum, T. Starch, gluten and allergen content (n = 3, 3, and 2, respectively) are mean ± SD. Starch and allergen contents did not show statistical differences by Tukey-Kramer method (P < 0.05).
Protein content in flour samples was determined using the Kjeldahl method [18] by Japan Food Research Laboratories. The average value from two biological replicates was reported. MO3M flour exhibited a protein content of 16.2 g/100 g, approximately double that of MO3 flour (8.2 g/100 g) (Table 1). This difference was consistent with other harvest year and that protein content of MO3M was 18.1 g/100 g and MO3 was 9.1 g/100 g in the subsequent harvest year. MO3M displayed a significantly higher protein content compared to cake flour (8.3 g/100 g; https://fooddb.mext.go.jp/), bread flour (11.8 g/100 g; https://fooddb.mext.go.jp/) and Mochihime flour (9.5 g/100 g) [19]. It is important to note that the protein content of commercially available bread, cake, and Mochihime flours varies depending on factors such as fertilizer application [20] and milling efficiency.
Given the high protein content of MO3M flour determined by the Kjeldahl method, its gluten-forming ability was assessed following previously described methods [21] and protocols approved by the American Association of Cereal Chemists (https://www.cerealsgrains.org/resources/Methods/Pages/38Gluten.aspx). MO3 flour yielded almost no gluten, whereas MO3M exhibited a gluten content (43.3 %) comparable to bread flour (43.1 %) and Mochihime flour (41.2 %) (Table 1). One hypothesis to explain the observed results is that diploid wheat varieties might possess higher protease activity compared to hexaploid lines, since protease activity interferes with the gluten formation [22]. This could have led to partial degradation of gluten-forming proteins in MO3 during gluten isolation, rendering them incapable of gluten network formation. Conversely, the higher protein content (double that of MO3) in MO3M may have provided sufficient substrate despite potential proteolysis, allowing for gluten formation. Alternatively, interactions with water-insoluble components such as fiber and lipids may have influenced gluten content. Studies have shown that these components can interact with gluten proteins [23, 24]. For examples, whole wheat flour rich in fiber inhibits gluten formation during bread dough preparation, unlike plain flour [25]. Since MO3 potentially contained high levels of fiber and lipid, this could explain the observed decrease in gluten yield. The gluten-forming ability of MO3M suggests its potential as an ingredient for leavened bread (Table 1).
To investigate the potential influence of lipid and fiber content on gluten formation, flour samples were analyzed by Japan Food Research Laboratories. Single-replicate analyses were performed using the acid digestion method for lipid content and gravimetric methods for fiber content. MO3M flour exhibited a slightly higher lipid content (2.1 g/100 g) compared to MO3 (1.6 g/100 g), commercially available cake and bread flours (1.5 g/100 g; https://fooddb.mext.go.jp), and Mochihime flour (1.4 g/100 g) (Table 1). MO3M exhibited a significantly higher fiber content (9.5 g/100 g) compared to MO3 (6.1 g/100 g), representing a 1.5-fold increase. This value was also over three times higher than that of cake flour (2.5 g/100 g; https://fooddb.mext.go.jp), bread flour (2.7 g/100 g; https://fooddb.mext.go.jp), and Mochihime flour (2.7 g/100 g). MO3, which displayed minimal gluten formation, also possessed higher fiber content than cake, bread, and waxy hexaploid wheat varieties. These findings suggest that gluten formation of MO3 was possibly inhibited by high fiber content, but not in MO3M because of high protein content (Table 1). Gluten formation likely requires a fine balance between protein and fiber contents [23].
To investigate the mechanisms underlying the high gluten-forming ability of MO3M, its protein composition was examined using SDS-PAGE. Total protein was extracted from equal flour weights using 30 volumes of a denaturing extraction buffer (0.125 M Tris-HCl, pH 6.8, 8 M urea, 4 % SDS, 5 % β-mercaptoethanol) as previously described [26] (Fig. 1A, left panel). Subsequently, alcohol-soluble seed storage proteins were selectively extracted using 60 % ethanol containing 0.5 % β-mercaptoethanol (Fig. 1A, right panel). Residual, alcohol-insoluble proteins were then extracted using the same denaturing extraction buffer (Fig. 1A, middle panel). Equal volumes of protein extracts from the total, residual, and alcohol-soluble fractions were loaded onto the SDS-PAGE gel. The CBB-stained gel was visualized using a LAS-4000 gel imager (FUJIFILM Corporation, Tokyo, Japan). The protein banding patterns observed on the SDS-PAGE gel differed between the hexaploid (bread, cake, and Mochihime flours) and diploid (MO3 and MO3M) wheats. However, the protein profiles of MO3 and MO3M displayed a high degree of similarity (Fig. 1A). Three major high molecular weight glutenin subunits were observed in the hexaploid wheats, but only one major band was observed in diploid wheat lines. In addition, a greater number of low molecular weight glutenin and γ-gliadin subunits were observed in the alcohol soluble fraction extracted from hexaploid wheats (Fig. 1A). Intensity of ω-gliadin in the alcohol soluble protein was higher in cake and bread flour than Mochihime (Fig. 1A).
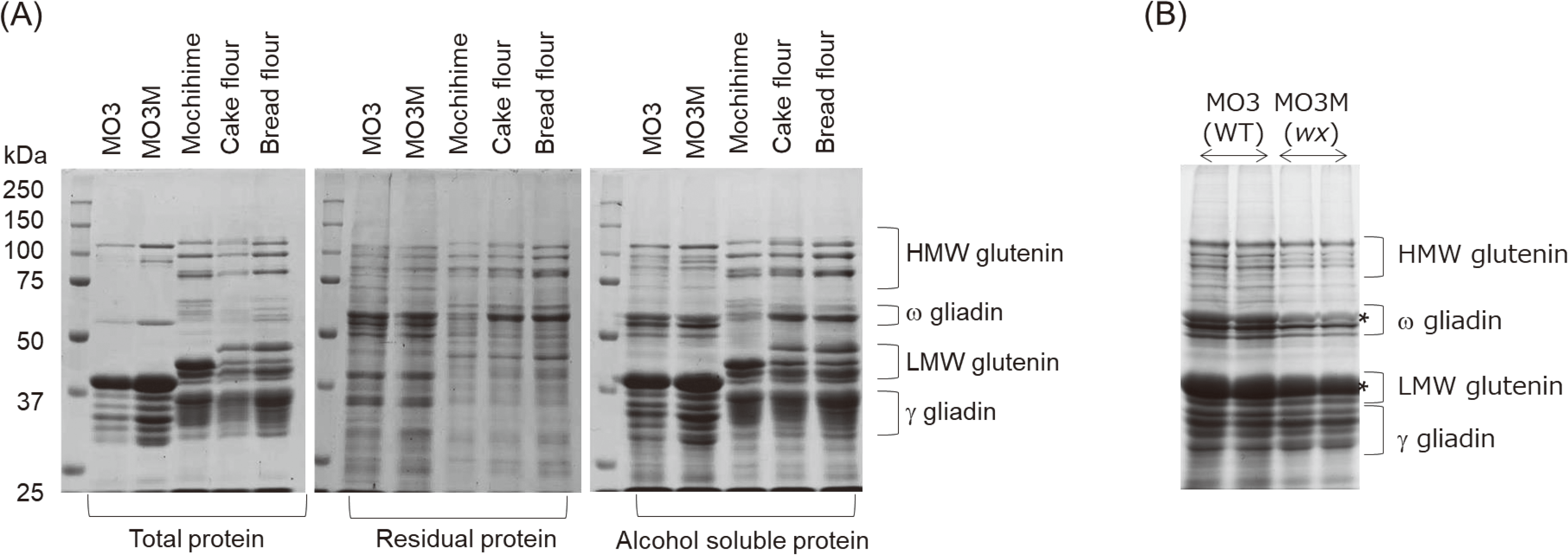
Consistent with the Kjeldahl method revealing the highest protein content in MO3M flour, SDS-PAGE also revealed the most intense protein bands for this line (Fig. 1A). This might be partly due to thinner grains compared to MO3. Consequently, the flour of MO3M may have a higher relative protein concentration since storage proteins are enriched in sub-aleurone layer [26]. Mochihime flour exhibited a similar trend, possessing both higher protein and fiber content than cake flour (Table 1).
To see the abundance of storage proteins within the total protein, equal amounts of protein extract (corresponding to 60 μg protein based on the Kjeldahl analysis) were loaded onto the SDS-PAGE gel. The CBB-stained gel image captured using the LAS-4000 imager was then quantified using MultiGauge software (FUJIFILM Corporation) (Fig. 1B). SDS-PAGE analysis revealed that the intensities of protein bands corresponding to ω-gliadin (an allergen protein) and low molecular weight glutenin subunits were significantly lower (approximately 60 %) in MO3M compared to MO3 (Fig. 1B).
To investigate potential changes in allergen content, the levels of wheat allergens in flour were quantified using the FASTKIT Elisa ver. III wheat (Nipponham Foods Ltd., Osaka, Japan), following the manufacturer's instructions. Briefly, flour samples were extracted using the provided buffer and then diluted 2,000-fold and 5,000-fold with the supplied dilution buffer. Aliquots of 100 μL of each diluted extract were transferred to the provided 96-well plate. Subsequently, 100 μL of biotin-conjugated antibody solution was added to each well, followed by a 30-minute incubation period. Following the washing step, 100 μL chromogenic solution was added to each well and incubated for 20 min. The reaction was then terminated by adding 100 μL of stop solution. The absorbance of the samples was measured at 450 and 600 nm using a plate reader. The difference between these absorbance values (450-600 nm) was calculated and used for a four-parameter logistic curve analysis with the provided standard material. Finally, the average values from each dilution were determined and used for further analysis (Table 1). Quantification analysis revealed that MO3M contained 97 mg/g of allergens, which was slightly lower compared to bread (109 mg/g) and Mochihime flour (116 mg/g). Despite the high protein content of MO3M, its allergen content was statistically not significant from others by Tukey-Kramer method (P < 0.05). This observation might be attributed to the reduced levels of ω-gliadin in MO3M (Fig. 1B). ω-gliadin is known as a major wheat allergen [27, 28]. However, it is important to consider that even small quantities of allergen can trigger severe reactions in susceptible individuals. Therefore, a modest reduction in allergen content, as observed in MO3M, may not be sufficient to ensure safety for wheat-allergic consumers.
Wheat flour's rheological properties influence its behavior during food production. These properties are directly affected by the composition and proportions of starch and protein within the flour. To evaluate the rheological properties of MO3 and MO3M flours, a dough preparation and measurement procedure was performed. Two and a half grams (2.5 g) of each flour sample were weighed and mixed with 1.5 mL of distilled water. The resulting dough was kneaded until uniform, then sandwiched between the plates of a rheometer consisted of 25 mm diameter parallel plates with a gap size of 2 mm. An initial oscillatory strain of 0.05 % at a frequency of 1 Hz was applied using a rheometer (Rheosol G-3000, UBM Co., Kyoto, Japan). The temperature was then increased from 28 to 100 °C at a constant heating rate of 2 °C/min while the storage modulus was monitored. Bread flour exhibited an increase in storage modulus starting at approximately 40 °C, reaching a maximum value (4.0 × 105 Pa) above 80 °C. Compared to bread flour, cake flour exhibited a lower storage modulus of up to 60 °C. Following this, a sharp increase was observed, with the modulus reaching 2.5 × 105 Pa at temperatures above 90°C. In contrast, Mochihime flour displayed a gradual rise in storage modulus starting at 45 °C. The maximum value of 1.0 × 105 Pa at 100 °C remained lower compared to both bread and cake flours. The storage modulus of MO3 did not significantly increase until 65 °C, and then increased rapidly up to 80 °C, reaching 2.0 × 105 Pa at 100 °C. Finally, MO3M exhibited the highest initial storage modulus. This value remained relatively constant until 65 °C. However, its maximum storage modulus (1.0 × 104 Pa at >80 °C) was significantly lower compared to the other lines. These results suggest that amylose content plays a crucial role in storage modulus at high temperatures, with its absence leading to lower values. Conversely, higher protein content appears to contribute to increased storage modulus at lower temperatures (below 40 °C) (Fig. 2). Overall, MO3M showed smallest change in storage modulus in a wide range of temperature.

To investigate the potential influence of starch structure on rheological properties, the chain length distribution of starch was analyzed in the flours used for the rheological measurements. Starch was isolated from the flours using the cold alkaline method [29, 30] and then debranched with isoamylase preparation from Pseudomonas amyloderamosa (Nagase Viita Co., Ltd., Okayama, Japan). Analysis of amylopectin chain length distribution was performed using capillary electrophoresis (AB Sciex) following established protocols [29]. As shown in Fig. 3A, the initial examination revealed a similar distribution pattern for endosperm starch isolated from MO3, MO3M, and Mochihime flours, consistent with findings reported in another study [14]. To elucidate subtle differences, subtraction curves were generated by subtracting the Mochihime profile (Fig. 3B). Although the overall patterns remained comparable, Mochihime displayed a slight enrichment in short amylopectin chains (degree of polymerization, DP 6-10) (Fig. 3B).

Given the similar amylopectin structures observed for MO3, MO3M, and Mochihime flours (Fig. 3), a gel-filtration chromatography method with a refractive index detector was employed to determine the precise apparent amylose content. The chromatography system utilized Toyopearl HW55S-50S × 3 columns and debranched starch prepared according to a previously described method [31]. As illustrated in Fig. 4, fraction I of MO3 displayed an amylose content of 30.8 %, while MO3M (1.7 %) and Mochihime (4.7 %) exhibited significantly lower values. However, a small amount of amylose was detectable in both MO3M and Mochihime flours. Previous studies relied on methods such as Sephadex-75 gel-filtration chromatography and iodine staining for amylose content determination [14]. However, these methods offer limited accuracy. The present study employs a more precise approach, revealing the accurate amylose content (Fig. 4). The amylose detected in waxy wheats is presumably synthesized by GBSSII which is predominantly expressed in vegetative tissues [7].

Considering the similarity in starch structure between MO3M and Mochihime, the observed differences in storage modulus likely stem from variations in protein content. High molecular weight glutenin subunits, in particular, are well known for their contribution to dough elasticity [32]. Additionally, factors like insoluble fibers (e.g., cellulose), can hinder elasticity [33], whereas water-soluble glucans (e.g., β-glucan), can promote it [34]. Consequently, MO3M dough might exhibit increased firmness, potentially leading to crumblier or softer baked/cooked products, depending on the application. However, it is important to consider that water content and other ingredients significantly influence the final textural properties of cookies, bread, and noodles. To gain a more comprehensive understanding of these rheological properties, further analysis would be beneficial.
Mochihime, a waxy hexaploid wheat variety, finds applications in various food products like noodles, dumplings, bread, and confectionery [17]. It is particularly valued for imparting a desirable chewy texture to these foods. However, Mochihime also exhibits softening characteristics and reduces adhesiveness, making it easier to chew and swallow. This property proves beneficial for elderly individuals with swallowing difficulties and young children during weaning, as it reduces the risk of choking [35, 36]. MO3M exhibited a higher storage modulus compared to other wheat lines at lower temperatures. This likely translates to firmer dough with improved handling properties during shaping and slicing. MO3M displayed a significantly lower storage modulus at high temperatures (>70 °C). Based on these rheological properties and comparison to Mochihime (Fig. 2), MO3M is expected to contribute a softer and less adhesive texture in food applications while maintaining chewiness. In addition, MO3M showed smallest change in storage modulus in a wide range of temperature, which may be useful characteristics for food processing. Therefore, the unique characteristics of the diploid waxy mutant MO3M, as revealed in this study, offer valuable insights for practical applications. In rice breeding, backcrossing a waxy mutant with a large-seeded cultivar successfully improved seed weight [37]. This approach suggests that backcrossing MO3M with a wheat cultivar known for large seeds could effectively address its low seed weight. Furthermore, established cleaved amplified polymorphic sequence markers specific to MO3M can significantly accelerate breeding efforts. These markers would allow the selection of heterozygous F1 seedlings during backcrossing with elite wheat cultivars [15].





