2013 Volume 52 Issue 1 Pages 58-63
2013 Volume 52 Issue 1 Pages 58-63
This study investigated the mechanism by which the strength and weakness of exercise stress affects the skin symptoms of atopic dermatitis (AD). Specific pathogen-free (SPF) and conventional NC/Nga mice were used. Conventional mice, but not the SPF, spontaneously develop dermal symptoms similar to that of patients with AD. There were two types of stress, mild (20 m/min for 60 min) or strong exercise (25 m/min for 90 min), using a treadmill four times per day. The symptom of the conventional group were strongly exacerbated by strong exercise but ameliorated by mild exercise. The plasma concentrations of α-melanocyte stimulating hormone (α-MSH) and the expression of melanocortin receptor-1 in skin elevated after strong exercise but decreased after mild exercise. The plasma levels of β-endorphin and the expression of µ-opioid receptor in skin were increased by mild exercise. In addition, the expression of prohormone convertase (PC) 1/3, PC2 and carboxypeptidase E (CPE) in pituitary gland were higher in the conventional group than in the SPF group. The level of PC2 was suppressed by mild exercise in the conventional groups, and elevated further by strong exercise. The level of PC1/3 becomes higher with the increase of the exercise load. On the other hand, the expression of the CPE was further increase by mild exercise but suppressed by strong exercise. These observations suggested that exercise-induced stress significantly affect the symptoms of AD in a pivotal manner depending on the levels of α-MSH and β-endorphin, and the expression of pituitary PC2 and CPE.
Atopic dermatitis (AD) is a chronic-intermittent eczematous skin disease associated with dry skin, inflammation and pruritus.(1,2) Psychological stress affects the symptoms of allergic diseases including AD.(3–7) The activation of the hypothalamic-pituitary-adrenal axis (HPA axis) and/or the sympathetic nervous system may thus play an important role in the stress-induced modulation of allergic diseases.(3,8) The activation of these systems induces changes in the cytokine balance in the circulation and at the site of inflammation.(3,9–15) These observations suggest that an appropriate response to various stresses through the HPA axis and/or autonomic nervous system is necessary for the regulation of immunological systems required for the survival animals.(16,17)
The secretion of corticotrophin-releasing hormone (CRH) from the hypothalamus increases the synthesis of proopiomelanocortin (POMC) in the pituitary lobe. POMC is the precursor for adrenocorticotropic hormone (ACTH), melanocyte stimulating hormone (MSH), and β-endorphin; these hormones have numerous activities, including regulation of the immune response. There have been a number of reports concerning the participation of circulatory POMC-derived peptides in the allergic response.(18,19) Furthermore, a peptide hormone derived from POMC induces the change of the allergic symptoms caused by the strength and weakness of the stress. For example, in the pollen-antigen sensitized mice, a mild restraint stress increased the secretion of the ACTH and suppressed the onset of pollinosis.(20)
NC/Nga mice, an inbred strain of Japanese origin, have the healthy skin under the specific pathogen-free (SPF) environment. But when they are kept long under conventional condition, they develop chronic dermatitis and IgE hyperproduction,(21,22) which may be due to mite infection and allergy.(23) Spontaneous scratching, an itch-associated response, gradually increases with the worsening of dermatitis in NC/Nga mice kept under conventional conditions, but not under the SPF condition.(22) Theses features are similar to those of human AD and therefore NC/Nga mice have been used as AD model mice. Strong stress exacerbates skin symptoms in AD, while mild stress reduces such symptoms. α-MSH and β-endorphin play an important role in this reaction.(24)
This report describes the effects of mild and strong stress elicited by treadmill exercise on the peptide hormones derived from the pituitary gland and AD-like symptoms in the NC/Nga mice.
Conventional and specific pathogen-free (SPF) NC/Nga male mice (7 weeks old) were purchased from SLC (Hamamatsu, Japan). They were housed in rooms with a 12-h light/12-h dark cycle, and all animals were allowed free access to laboratory chow (CE-2, Oriental Yeast Co., Tokyo, Japan) and water ad libitum during the experiments. These animals were subjected to experiments according to the animal care regulations of Osaka City University Medical School. Conventional but not SPF mice spontaneously started to exhibit the symptoms characteristic to AD at their age of 7 weeks old. The mice were divided into six groups (n = 10) and used at same time. In addition, each experiment was repeated three times.
Voluntary and forced exercise protocolsThe details of the protocol are described in a previous article.(24) Animals were conditioned an exercise device by placing themon a resting treadmill for 10 min followed by running at 5 m/min for 10 min and then at 10 m/min for 10 min. The animals were placed in an up-hill inclined device (at 10°) on day 2, at a speed of 20 m/min for 60 min or 25 m/min for 90 min to give mild or strong stress, respectively. The latter conditions elicited strong fatigue (the subjects were unable to move due to exhaustion) while the former conditions provided only mild stress (no appreciable effect on their behavior) based on their performance analysis for the next 60 min. These exercise programs were carried out every other day (for a total of 4 exercise sessions).
Evaluation of the inflammatory scoreThe symptoms of dermatitis in these animals were evaluated on day 9, at their rostral skin and the severity of edema, erythema and hemorrhage was scored (0, none; 1, slight; 2, moderate; 3, severe) as described previously.(25)
Analysis of peptide hormones in plasmaUnder light ether anesthesia, blood samples were obtained by cardiac puncture 24 h after giving final exercise. Plasma samples were obtained by centrifugation and analyzed for peptide hormones. The plasma levels of ACTH, α-MSH and β-endorphin were determined by using ELISA kits according to manufacture’s instructions. ELISA kits for ACTH, α-MSH and β-endorphin were obtained from Phoenix Pharmaceuticals (Belmont, CA).
Preparation and staining of pituitary samplesThe pituitary specimens were fixed in phosphate-buffered paraformaldehyde (4%), embedded in frozen Tissue-Tek, OCT compound, and cut into 5 µm thick sections. The sections of the pituitary gland were washed in PBS and then were subsequently incubated overnight at 4°C with rabbit anti prohormone convertase-1/3 (PC1/3; 1:100) polyclonal antibody (MILLIPORE, Temecula, CA), rabbit anti PC2 (1:100) polyclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) or rabbit anti carboxypeptidase E (CPE; 1:100) polyclonal antibody (GeneTex Inc., Irvine, CA), in order to determine the expression of PC1/3, PC2 and CPE. The sections were then washed in PBS and incubated at room temperature for 2 hours with FITC-conjugated anti-rabbit immunoglobulin or TRITC-conjugated anti-rabbit immunoglobulin (1:30; Dako Cytomation, Glostrup, Denmark). The expression levels of PC1/3, PC2 and CPE were evaluated immunohistochemically using a fluorescent microscope.
Preparation and staining of skin samplesThe skin specimens were fixed in phosphate-buffered paraformaldehyde (4%), embedded in frozen Tissue Tek, OCT compound and cut into 5 µm thick sections. The sections of skin was washed in PBS and subsequently incubated overnight at 4°C with rabbit anti melanocortin-1 receptor (MC1R; 1:100) polyclonal antibody (CHEMICON, CA) or rabbit anti µ-opioid receptor (1:100) polyclonal antibody (Enzo Life Sciences Inc., NY), for the analysis of the expression of MC1R and opioid receptor. The sections were then washed in PBS and incubated at room temperature for 2 h with FITC-conjugated anti-rabbit immunoglobulin or TRITC-conjugated anti-rabbit immunoglobulin (1:30; Dako Cytomation, Denmark). The expressions of MC1R and µ-opioid receptor were evaluated immunohistochemically using a fluorescent microscope.
Western blot analysis of pituitary samplesThe pituitary samples were homogenized in a lysis buffer containing 0.5% Nonidet P-40, 10% glycerol, 137 mM NaCl, 2 mM ethylenediamine tetraacetic acid and 50 mM Tris-HCl buffer (pH 8.0). After centrifugation at 8000 × g for 10 min, the supernatant fractions were separated and stored at –80°C until use. The stored pituitary specimens were subjected to 10% polyacrylamide gel electrophoresis (PAGE) in the presence of 0.1% SDS. The electrophoresed proteins in the gel were transferred to an Immobilon membrane (Millipore, Bedford, MA). The membrane was blocked with 5% skim milk at 4°C for over-night and subsequently incubated with primary antibodies against PC2 (1:1000) polyclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) at 25°C for 1 h and then with horseradish peroxidase-conjugated secondary antibody (Dako Cytomation, Glostrup, Denmark). Immune complexes thus formed were detected with Enhanced chemiluminescence (ECL) regents (GE Healthcare Bio-Sciences, Piscataway, NJ).
Statistical analysisAll data are presented as the mean ± SD derived from 10 animals. The results obtained from the two animal groups were analyzed by either Student’s t test or ANOVA using a computer software package. Differences were considered to be significant when p<0.05.
In our previous study,(24) conventional mice started to exhibit the symptoms characteristic of AD including edema, erythema and hemorrhage on their rostral skin. The dermal symptom was ameliorated in the animal group that received mild exercise. In contrast, the symptoms deteriorated in the animal group that received strong exercise. The symptoms of dermatitis were not apparent in the SPF group even if they were treated with strong exercise. In addition, exercise stress increased the plasma levels of α-MSH in the SPF group irrespective of the difference in the strength because α-MSH is derived from the N-terminal domain of ACTH. The plasma levels of ACTH were significantly higher in the conventional group than in the SPF group. However, the levels remained unaffected by exercise. In contrast, the elevated levels of α-MSH were suppressed by mild exercise and elevated further by strong exercise. It should be noted that POMC, which is associated with ACTH/α-MSH and β-endorphin within its molecule, is also synthesized in dermal keratinocytes, particularly when the skin is exposed to a variety of stress including ultraviolet B and inflammation.(26,27) Therefore, this study measured the dermal expression of β-endorphin at the site of inflammation. The plasma levels of β-endorphin increased markedly when the symptom similar to atopic dermatitis became apparent in the conventional group. Although the plasma levels of β-endorphin were not affected by exercise in the SPF group, the levels were further increased by mild exercise but suppressed by strong exercise in the conventional group.
Effect of exercise on the expression of MC1R (Fig. 1) and µ-opioid receptor (Fig. 2) in the skinThe exercise stress increased the expression of MC1R and the µ-opioid receptor in the SPF group irrespective of the difference in the strength. The expression of MC1R and µ-opioid receptor was significantly higher in the conventional group than in the SPF group. However, the levels remained unaffected by exercise. In contrast, the elevated levels of MC1R were suppressed by mild exercise and elevated further by strong exercise. On the contrary, the expression of the µ-opioid receptor was further increase by mild exercise but suppressed by strong exercise in the conventional group.

The expression of MC1R in the dorsal skin of SPF (D, E, F and H) and conventional (A, B, C and G) NC/Nga mice. The data show one typical experiment from 10 animals. Scale bar = 100 µm. Values are presented as the mean ± SD derived from 10 animals. *p<0.05 in comparison to no exercise SPF or conventional NC/Nga mice.

The expression of µ-opioid receptor in the dorsal skin of SPF (D, E, F and H) and conventional (A, B, C and G) NC/Nga mice. The data show one typical experiment for 10 animals. Scale bar = 100 µm. Values are presented as the mean ± SD from 10 animals. *p<0.05 in comparison to no exercise SPF or conventional NC/Nga mice.
The production of the peptide hormones, ACTH, α-MSH and β-endorphin requires the proteolytic processing of POMC, which is hypothesized to utilize dual cysteine and subtilisin-like protease pathways, including the secretory vesicle cathepsin L pathway and prohormone convertase pathway. The expression of PC1/3, PC2 and CPE were higher in the conventional group than in the SPF group. The elevated level of PC2 was suppressed by mild exercise in the conventional groups, and elevated further by strong exercise. The elevated level of PC1/3 becomes higher with the increase of the exercise load. On the other hand, the expression of the CPE was further increase by mild exercise but suppressed by strong exercise.

The expression of PC1/3 in the pituitary gland of SPF (D, E and F) and conventional (A, B and C) NC/Nga mice. The data show a typical experiment from 10 animals. Scale bar = 10 µm.
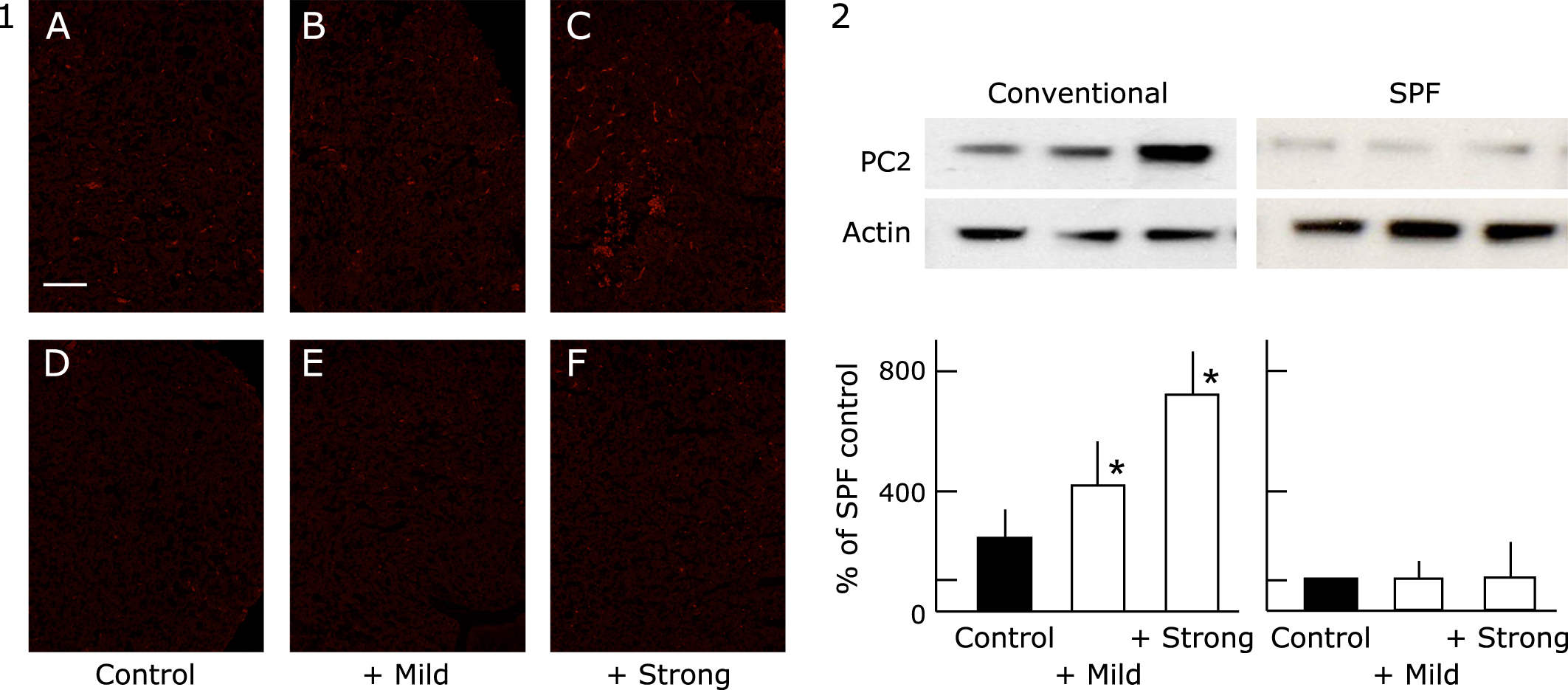
The expression of PC2 in the pituitary gland of SPF (D, E and F) and conventional (A, B and C) NC/Nga mice by immunohistochemistry (Fig. 4-1) and by western blot analysis (Fig. 4-2). The data show a typical experiment from 10 animals. Scale bar = 10 µm. Values are mean ± SD derived from 10 animals. *p<0.05 in comparison to no exercise SPF or conventional NC/Nga mice.

The expression of CPE in the pituitary gland of SPF (D, E and F) and conventional (A, B and C) NC/Nga mice. The data show a typical experiment from 10 animals. Scale bar = 10 µm.
This present study demonstrated that AD like symptom of the conventional mice was exacerbated by strong exercise but ameliorated by mild exercise. In addition, the levels of neuropeptides in plasma were significantly increased in the exercise stress load group. However, the level of α-MSH in conventional mice was decreased with mild stress in comparison to a non-stress control group and increased in strong stress. In contrast, the level of β-endorphin in conventional mice was decreased in strong stress group in comparison to the non-stress control group and increased in mild stress group.
POMC and its related peptide hormones, such as ACTH, MSH and β-endorphin, play key roles in stress responses and immunological modulation.(28–30) Although the plasma levels of ACTH were elevated markedly in the conventional group, they were unaffected either by strong or mild exercise, despite the marked effects of these different extents of exercise on the symptom of dermatitis. Therefore, the stress elicited by the symptom of dermatitis, such as pruritus, might be strong enough to increase ACTH levels maximally in plasma. Plasma levels of α-MSH were also higher the conventional group than in SPF group. However, the plasma levels were increased further by strong exercise but decreased by mild stress. This observation suggested that α-MSH-induced signaling might participate in the exacerbation of the symptoms of AD. In fact, α-MSH interacts with MCRs (especially MC1R) on a variety of leukocytes, thereby modulating inflammatory reactions induced by endotoxin and related cytokines including IL-1, TNF-α and IFN-β.(31–34) α-MSH enhances the production of TGF-β1.(35) Therefore, the TGF-β/TGF-β-R signaling pathway might activate POMC system to increase plasma levels of α-MSH, thereby exacerbating the symptoms of AD.
On the other hand, plasma β-endorphin also serves as an independent and important factor influencing pruritus in AD. Opioid peptides and their µ-specific receptors modulate the function of calcium channels specifically on unmyelinated C-fibers of the central nervous system, and thus, they are probably involved in the central itch-regulatory mechanism.(36) Some reports suggest that endogenous opioid peptides may be involved as central mediators of itch.(37,38) β-endorphin, belonging to the endogenous opiate family, is generated upon stimulation of the pituitary-adrenal axis after stress.(39,40) The present study also demonstrated that, although plasma levels of β-endorphin were significantly higher in the conventional group than in the SPF group, mild exercise increased the plasma level with a concomitant amelioration of dermatitis, while strong exercise decreased the plasma level and exacerbated the symptoms. These results suggest that the different strength of the exercise modulated plasma levels of α-MSH and β-endorphin in a pivotal manner and affected the symptom of dermatitis.
How is the control of ACTH, MSH and β-endorphin accomplished? POMC is synthesized mainly in the neurointermediate lobe of the pituitary gland(41) and is the precursor of ACTH and other peptides. Two mammalian prohormone convertases, PC1/3 and PC2, cleave POMC and other peptide precursors.(42,43) PC1/3 mediates the initial cleavage at paired basic residues into ACTH and β-lipotropic hormone, whereas PC2 converts ACTH and β-LPH into α-MSH and β-endorphin, respectively.(42,43) In addition, β-endorphin finally becomes the active form by cutting one of the amino acid residues of the c-terminal using CPE.(44) All expressed PC1/3, PC2 and CPE were increased by mild exercise in the current study, and increases in expressed PC1/3 and PC2 were seen with strong exercise. The strong stress derived increase of the expression of PC2 and, as a result, the increased α-MSH and exacerbated the skin symptoms. On the other hand, mild stress increased expression of CPE and increased endorphins and reduces the skin symptoms.
The current results showed the hormonal regulation supporting the phenomenon that mild exercise is effective for AD. This suggests the possibility of new innovative drug development to prevent AD under the knowledge of this mechanism.
ACTH; adrenocorticotropic hormone, α-MSH; α-melanocyte stimulating hormone, CPE; carboxypeptidase E, PC; prohormone convertase, POMC; proopiomelanocortin
The authors have no conflict of interest.