2013 年 52 巻 2 号 p. 128-132
2013 年 52 巻 2 号 p. 128-132
The reactions of three α-oxoaldehydes (methylglyoxal, glyoxal, and pyruvic acid) with hydroxyl radicals generated by sonolysis of water were investigated using an electron spin resonance (electron paramagnetic resonance) spin-trapping method, and their reaction kinetics were investigated. It is apparent from our experimental results that methylglyoxal exhibits the highest reactivity of the three α-oxoaldehydes. These α-oxoaldehydes can react with hydroxyl radicals faster than other well-known antioxidants can. The reactivity of hydroxyl radicals is higher than that of hydrogen peroxides.
Methylglyoxal (MG), glyoxal (GO), and 3-deoxyglucoson are well-known precursors of advanced glycation end-products (AGEs). They may cause damage to cells(1) and their concentrations in blood are higher for dialysis patients and for Chronic kidney disease (CKD) patients than those for healthy persons.(2–4) In particular, MG is known to be a cytotoxic α-oxoaldehyde and its cytotoxicity depends on the enhancement of oxidative stress, which is caused mainly by reactive oxygen species (ROS). In some cases, MG is directly oxidized to pyruvic acid (PA) by methylglyoxal dehydrogenase, and then this PA enters the tricarboxylic acid (TCA) cycle.(5–7) It has been postulated that the production of ROS is increased in various tissues under diabetic conditions.(8) Superoxide anions (O2−), hydrogen peroxide (H2O2), hydroxyl radicals (HO•), and singlet oxygen (1O2) are involved in ROS, but it is not clear which ROS can cause pathological disorders. O2− may be eliminated as a causative candidate because of its low reactivity. The production of H2O2 through glucose autoxidation has also been reported to be associated with cataract formation as a diabetic complication.(9) HO• is known to have the strongest oxidizing ability among ROS. The oxidizing abilities of H2O2 and HO• for AGEs are, therefore, important factors in understanding the initiation mechanisms of diabetic complications. Recently, we focused on the reaction between HO• and two α-oxoaldehydes (MG and GO) to clarify whether HO• or H2O2 is associated with diabetic complications. As a model reaction for oxidative damage that may cause diabetic complications, we have already reported that free radicals are generated in vitro via a non-enzymatic reaction between H2O2 and MG or GO.(10)
HO• is usually generated by the reaction of Fe(II) ions with H2O2 (Fenton reaction) or by ultraviolet (UV) irradiation of H2O2, but the substrates that may react with HO• are affected by UV or by the Fe(III) ions.(11,12) The sonolysis of aqueous solutions is a powerful alternative method for generating HO•. When aqueous solutions are irradiated with ultrasound, HO• and hydrogen atoms are generated. This free-radical generation is confirmed by electron spin resonance (ESR; electron paramagnetic resonance) and spin-trapping experiments.(13) In our recent study, these free radicals were generated by high-frequency ultrasound irradiation (1650 kHz) of pure water using an ESR spin-trapping technique.(14,15) The free-radical-generation patterns differed, depending on the dissolved gases. Only HO• could be detected with the O2 gas or H2 gas bubbling or, that is, in the presence of dissolved oxygen. In the present study, the reactions of HO• generated by the sonolysis of water or H2O2 with two α-oxoaldehydes (MG and GO) and PA were kinetically studied using an ESR spin-trapping technique (the chemical structures are shown in Fig. 1). The initiation of diabetic metabolism was then discussed in terms of the reactivities of ROS with AGEs.

Chemical structures of methylglyoxal (MG), glyoxal (GO), and pyruvic acid (PA).
5,5-Dimethyl-1-pyrroline N-oxide (DMPO) was purchased from Labotec (Tokyo, Japan). MG, GO, PA, and H2O2 were supplied by Wako Pure Chem. Ind. Ltd. (Osaka, Japan). 4-Hydroxy-2,2,6,6-tetramethylpiperidine (TEMPOL) was purchased from Sigma Aldrich (St. Louis, MO). All the other reagents were of the highest commercially available purity.
Experimental deviceAn ultrasound device that generates 1650 ± 50 kHz ultrasound with an acoustic intensity of 30 W cm−2 was kindly supplied by the Nimo Co. (Nagoya, Japan). The ESR spectra were measured using an X-band ESR spectrometer (JES-FA-100, JEOL, Tokyo, Japan).
MethodMG, GO, and PA were dissolved in water at concentrations of 3–10 M. DMPO was diluted with ultrapure water to a 1.2 M concentration. A glass tube (diameter 15 mm, length 85 mm, and thickness 0.8 mm) containing α-oxoaldehyde (5–150 µl), DMPO (50 µl), and ultrapure water (0–145 µl) was placed in the ultrasonic device and then irradiated by ultrasound for 1 min. After the ultrasound irradiation, the aqueous solution of DMPO was transferred to a quartz cell for ESR spectrometry, and the ESR spectrum was recorded immediately. H2O2 was mixed with MG or GO aqueous solution containing DMPO and this solution was transferred to a quartz cell for ESR spectrometry. After 1 min of the reaction, the ESR spectrum was recorded. The ESR measurement conditions were as follows: field sweep, 330.550–340.500 mT; field modulation frequency, 100 kHz; field modulation width, 0.1 mT; sweep time, 2 min; time constant, 0.1 s; microwave frequency, 9.421 GHz; and microwave power, 1 mW. The proton or nitrogen hyperfine coupling constants (hfcc) and g-values of the DMPO spin adducts were determined using a computer anisotropic simulation system built in the ESR spectrometer. To calculate the concentrations of DMPO-OH, 10 µM TEMPOL was used as a standard sample for quantitative analysis, and the ESR spectrum of Mn2+ (which was present in the ESR cavity) was used as an internal standard. The concentration of OH• was determined using a Digital Data Processing (JEOL, Tokyo, Japan) system and the concentration of DMPO-OH was expressed as a micromolar value.
When an MG solution containing DMPO was irradiated with ultrasound at 1650 kHz, three types of spin adducts were observed in the ESR spectra of MgO doped with Mn2+ as an internal standard on both sides, as shown in Fig. 2. The concentration of DMPO was set at 300 mM since this concentration could trap all of the HO• generated by ultrasound irradiation of water, as described elsewhere.(14)
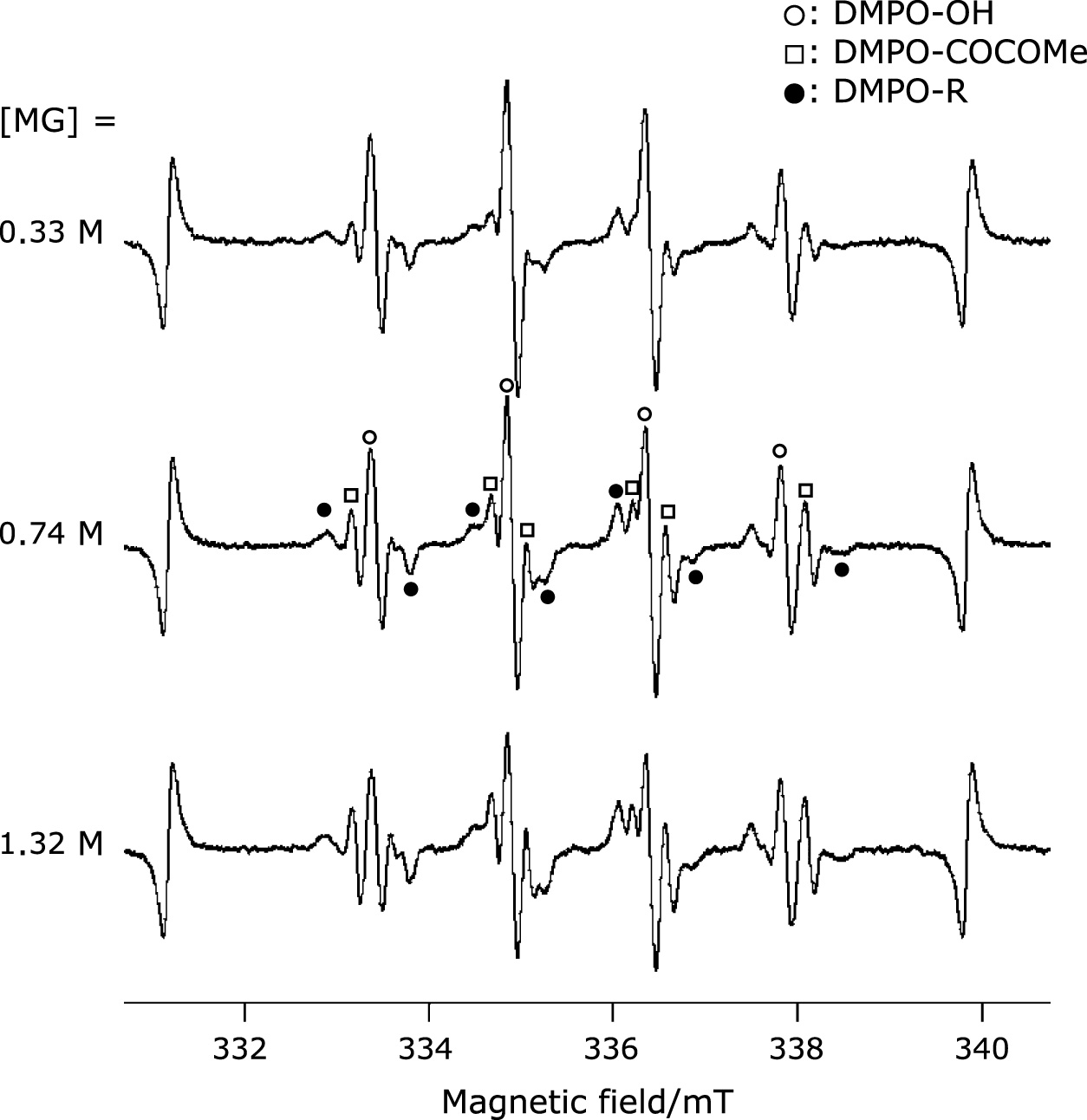
Electron spin resonance signals of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) spin adducts generated by ultrasound irradiation at 1650 kHz for 1 min of ultrapure water containing 300 mM of DMPO and different concentrations of methylglyoxal.
The spectral simulations in Fig. 2 were carried out using hfcc values of aN = 1.480 mT, aH = 1.468 mT, with g = 2.00635 (open circles) in a four-line spectrum, aN = 1.500 mT, aH = 1.880 mT, with g = 2.00635 (closed circles), and aN = 1.468 mT, aH = 2.220 mT, with g = 2.00650 (open squares). The spectra were assigned to the DMPO spin adducts typical of DMPO-OH,(16) DMPO-C(O)-C(O)-Me and DMPO-R, respectively, which led to the best fit to the experimental data. The intensity of the DMPO-OH signal decreased and that of the DMPO-C(O)-C(O)-Me slightly increased with increasing MG concentration. It was calculated that 28 µM of HO• were generated in 1 min from the concentration of DMPO-OH spin adducts without MG in the system. This concentration is best fit with the straight line which was reported in our previous paper where the HO• was generated in accordance with the linear proportion (y = 24.026x + 1.8382).(14) It was suggested that HO• can react with MG competitively or faster than DMPO can, resulting in a decrease in DMPO-OH spin adducts and an increase in DMPO-C(O)-C(O)-Me spin adducts as one of the reaction products. The reactivities of the α-oxoaldehydes were investigated by focusing on the concentration of DMPO-OH. The concentration of DMPO-OH is plotted against those of MG, GO, and PA in Fig. 3a. The concentration dependence of PA in PBS buffer solution at pH 7.4 was also plotted as PA is weak acid and the pH of the solution depends on its concentration.

(a) Effects of methylglyoxal (MG), glyoxal (GO), and pyruvic acid (PA) on the yield of 5,5-dimethyl-1-pyrroline N-oxide (DMPO)-OH. (◯): MG, (□): GO, (◇): PA, and (△): PA in PBS buffer at pH 7.4. (b) Plots of I0/Ix − 1 versus substrate concentration, where I0 is the initial DMPO-OH concentration and Ix is the DMPO-OH concentration at each substrate concentration. (●): MG, (■): GO, and (◆): PA.
The DMPO-OH concentration decreased in all cases with increasing substrate concentrations. There is no difference for PA systems between in water and in PBS buffer solution. These results indicate that these substrates can react with HO•, similar to the case of MG. The IC50 values were estimated in order to compare the reactivity of α-oxoaldehyde with HO• as shown in the following manner: IC50 values were determined from replots of (I0/Ix) – 1 against the substrate concentrations, where I0 and Ix are the concentrations of DMPO-OH without a substrate and with x M of substrate, respectively (Fig. 3b). Linear least-squares fitting of the data in the low concentration rage yielded straight lines, and the following equations were obtained:
MG: (I0/Ix) − 1 = 16.3[MG] (1)
GO: (I0/Ix) − 1 = 0.91[GO] (2)
PA: (I0/Ix) − 1 = 0.51[PA] (3)
The correlation coefficients for Eqs. (1), (2), and (3) are 0.998, 0.980, and 0.980, respectively.
The IC50 values were estimated as 0.061 M for MG, 1.100 M for GO, and 1.968 M for PA. The order of reactivity with HO• is found to be MG>GO>PA from the IC50 values. Then the apparent reaction constants (kx) where x is the name of the substrate, were calculated to compare the well-known HO• scavenger. The kx values were calculated using Eq. (4):
kx = kOH[DMPO]/IC50 (4)
where kOH is the rate constant of the reaction between DMPO and HO• and IC50 is the substrate concentration when DMPO-OH is at half its initial concentration.(17) The values of kx for kOH = 3.4 × 109 M−1 s−1 (16) and [DMPO] = 0.3 M were calculated and are listed in Table 1 with that of H2O2(14) and IC50 values. The calculated reaction rate constant for MG (kMG) is similar to the previously reported values of (4.3–8.1) × 109 M−1 s−1).(1,18) The estimation method using here is, therefore, available to discuss the reactivity with HO•. The kMG value is larger than kOH, and is the highest value of those for the three substrates.
| Substrate | IC50/M | kx/M−1 s−1 |
|---|---|---|
| MG | 0.061 | 1.7 × 1010 |
| GO | 1.100 | 9.3 × 108 |
| PA | 1.968 | 5.2 × 108 |
| H2O2 | — | 3.3 × 107 (14) |
This indicated that MG has highest reactivity with HO•. Furthermore, the reaction rates of these α-oxoaldehydes with HO• are higher than those of glycine [(1.5–4.0) × 109 M−1 s−1],(19) mannitol [(2.1 ± 0.58) × 109 M−1 s−1],(20) 7-hydroxycoumarin [6.1 × 109 M−1 s−1],(21) and hydroxycoumarin derivatives [(2.1–7.2) × 109 M−1 s−1](21) which are known as HO• scavengers. These findings suggest that α-oxoaldehydes can react with HO• faster than other well-known antioxidants can, and could not inhibit the autoxidation caused by free radicals generated from the reaction of α-oxoaldehydes with HO•.
The production of H2O2 is also associated with diabetic complications. It decomposes α-dicarbonyl compounds to the corresponding carboxylic acid through a Baeyer-Villiger reaction. It has also been reported that diacetyl compounds are formed from the dimerization of acetyl radicals derived from MG when H2O2 was added to MG.(22,23) The reactions of MG or PA with H2O2 were also investigated. When large amounts of H2O2 were reacted with MG in the presence of DMPO, two types of spin adducts were observed, and no acetyl radicals were observed in the ESR spectrum, as shown in Fig. 4. These spectra can be assigned to DMPO-OH and DMPO-C(O)-C(O)-Me, similar to the observations in Fig. 2. In contrast, only DMPO-OH was observed when PA was reacted with H2O2. It was apparent from these results that HO• was generated from the reaction of the α-oxoaldehyde with H2O2.

Electron spin resonance signals of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) spin adducts for the reaction of methylglyoxal with hydrogen peroxide (H2O2). [H2O2] = 1.25 M and [DMPO] = 300 mM.
The concentrations of DMPO-OH were plotted against those of two different substrates (MG and PA), as shown in Fig. 5. The concentration increased with increasing amounts of substrate in both cases. It is suggested that the generation of HO• is accelerated by MG and PA through these reactions. From these plots, the following equations are obtained:
MG: [DMPO-OH] = 2.00 × 10−5 × [MG] (r2 = 0.965) (5)
PA: [DMPO-OH] = 1.00 × 10−5 × [PA] (r2 = 0.985) (6)

Influence of hydrogen peroxide (H2O2) on the yield of 5,5-dimethyl-1-pyrroline N-oxide (DMPO)-OH. [H2O2] = 1.25 M and [DMPO] = 300 mM. Methylglyoxal: (◯) and pyruvic acid (◇).
The slopes of Eqs. (5) and (6) show that MG can react with H2O2 faster than PA can.
The apparent reaction constant (kMG–H2O2) of MG with H2O2 was then calculated. The time-course of DMPO-OH generation from the reaction of 1.25 M of H2O2 with 0.04 M of MG is shown in Fig. 6. The concentration of DMPO-OH increased with time. Moreover, the effect of reaction time was also the same in the case where PBS buffer at pH 7.4 was used as a solvent. Linear least-squares fitting of the data yields a line with a slope of 6 × 10−7 with a level of certainty, given by the correlation coefficient r2, 0.978. As the kMG–H2O2 value was calculated using Eq. (7):
d[DMPO-OH]/dt = kMG–H2O2[H2O2][MG] (7)

Time-dependent changes in the concentration of 5,5-dimethyl-1-pyrroline N-oxide (DMPO)-OH in the reaction of methylglyoxal (MG) with hydrogen peroxide (H2O2) in water(◯) and PBS buffer solution at pH 7.4(●). [MG] = 0.04 M, [H2O2] = 1.25 M, and [DMPO] = 300 mM.
where d[DMPO-OH]/dt = 6 × 10−7 (M min−1) = 1.0 × 10−8 (M s−1), [H2O2] = 1.25 M, and [MG] = 0.04 M, the kMG–H2O2 value was therefore calculated to be 2.0 × 10−7 M−1 s−1.
It is extremely low compared with that of the Fenton reaction, 2-8 × 104 M−1 s−1.(24) It is considered that this redox reaction is not the reaction that causes oxidative damage. However, its reaction rate constant is extremely low compared with that of the second-order reaction rate constant (kMG) between HO• and MG (1.7 × 1010 M−1 s−1), which is larger than those of other well-known antioxidants such as glycine and mannitol. The reaction of MG with HO• is faster than that with H2O2, that is, the generation of HO•. It is suggested that the oxidation product of MG may be one of the cause of the onset of oxidative damage. Since the reaction rate constants of GO and PA with HO• are almost the same as that of MG, the oxidation products from the reactions of GO and PA may be able to initiate oxidative damage.
As mentioned above, HO• may be one of the main candidates for causing the onset of oxidative damage. The mean concentrations of MG and GO in the blood of diabetic patients have been reported to be approximately 400 mM and 470 mM, respectively.(4) The reactions of MG and GO with HO• produced in the living body, shown in Eqs. (8) and (9), could initiate downstream autoxidation, resulting in the occurrence of diabetic complications.
HO• + CH3COCOH → CH3COC•O + H2O (8)
HO• + HCOCOH → HCOC•O + H2O (9)
We propose that diabetic complications occur as follows. The reaction products of α-oxoaldehydes, which are generated from the metabolization of glucose circulating in a living body, react with HO• produced in the immune cells. Oxidative damage may then cause diabetic complications. Further biological experiments are needed to elucidate our hypothesis.
It is apparent from our experimental results that MG exhibits the highest reactivity of the three α-oxoaldehydes (MG, GO, and PA) with HO•, and the reaction rates of these α-oxoaldehydes are faster than those of other well-known antioxidants. The reaction of MG with H2O2 generates HO• and the reactivity of MG with H2O2 is lower than that with HO•.
No potential conflicts of interest were disclosed.