2020 年 19 巻 4 号 p. 139-141
2020 年 19 巻 4 号 p. 139-141
Silicon carbide (SiC) exhibits super low friction in water environments. It has been well noticed that this super low friction property is caused by the generated lubricating layers through friction-induced chemical reactions. However, the chemical reactions at the friction interface have not been well clarified because it is difficult to observe the tribological processes at the friction interface in experiments. In the present study, we simulated the friction processes of SiC/SiC in water environments using reactive molecular dynamics simulations. It is interesting to observe that the hydrolysis reactions of Si-C and Si-Si bonds occurred, and however, the chemical reactions of C-C bonds hardly occur, indicating that Si atoms are preferentially oxidized and C atoms are not. The oxidation of Si atoms in SiC generates colloidal silica lubricating layers at the friction interface, being responsible for the super low friction of SiC.
Water lubrication is expected to be applied to manufacturing equipment used in clean environments, such as semiconductor and food manufacturing equipment because lubricating oil cannot be used in clean environments. It is known that silicon-based ceramics, such as silicon carbide (SiC) and silicon nitride, exhibits super low friction with a friction coefficient of less than 0.01 in water environments [1]. Furthermore, it has been well noticed that the super low friction property of silicon-based ceramics is caused by the generated lubricating layers through the friction-induced chemical reactions with water [2]. Therefore, it is important to elucidate the chemical reaction mechanisms of generating the lubricating layers, in order to implement water lubrication systems in a wide range of manufacturing fields in the future. However, it is difficult to observe the complicated friction-induced chemical reactions at the friction interface by experiments. On the other hand, reactive molecular dynamics (MD) simulation is a very effective method to reveal the chemical reaction dynamics in the friction processes and we have successfully applied it to diamond-like carbon (DLC) [3,4,5,6], DLC/SiC [7], SiO2 [8] and other friction systems. Other researchers also successfully applied the reactive MD method to a variety of frictions systems [9,10,11,12].
In the present study, reactive MD based sliding simulations on SiC/SiC friction system were performed and the chemical reactions at the sliding interface of SiC/SiC were analyzed to elucidate the generation mechanism of the lubricating layers which are responsible for the super low friction of SiC.
Figure 1 shows the sliding simulation model in the present study. A flat SiC substrate, SiC tip, and 13,000 H2O molecules were placed in the simulation cell to model the sliding interface in water environments. Here, amorphous SiC was used in both SiC substrate and SiC tip because the wear scar has an amorphous structure due to mechanical damage in general. The SiC substrate and SiC tip were terminated with OH groups and H atoms. The sliding simulations were performed under constant temperature (300 K) and constant volume (106 Å × 49 Å × 100 Å) condition. In the sliding simulation, the atoms in the lower part of the SiC substrate were fixed, whereas the atoms in the upper part of the SiC tip were slid at 100 m/s in the x-direction with a normal load of 78 nN in the z-direction. We employed a reactive force field (ReaxFF) [13] to simulate the chemical reaction dynamics at the sliding interface. This force field is based on the bond-order potential and then can describe the chemical reactions. Our developed ReaxFF parameter set in ref [7]. was employed. All the simulations were performed using our in-house MD program LASKYO.
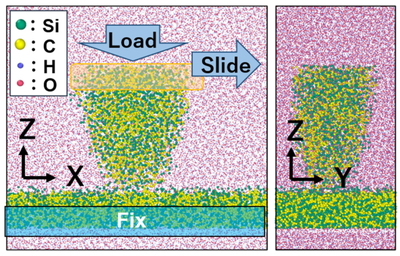
Sliding simulation model of SiC in water environments.
Figure 2 shows the snapshots of the sliding simulation. As shown in the upper left Figure, the SiC substrate and SiC tip terminated with OH groups and H atoms are employed at the initial model. The upper center Figure at 200 ps indicates that the SiC surface was oxidized by water molecules due to the friction-induced chemical reactions. Enlarged snapshot at lower center shows more detailed oxidized structure of SiC surface. Furthermore, it is interesting to see the generation of hydrocarbon molecules (e.g. CH4 and C2H6) at 200 ps. For example, enlarged lower left Figure shows the generation of C2H6 molecule. In addition, we also observed the generation of wear products of silicic acid and its diffusion into the water as shown in enlarged lower right Figure. The upper right Figure at 300 ps shows that the further oxidation of SiC substrate and SiC tip progressed.

Snapshots of the sliding simulation. H2O molecules are not shown for easy viewing. Lower left and center Figures show enlarged snapshots of the friction interface at 200 ps. Lower right Figure shows silicic acid observed at 200 ps.
To analyze the chemical reactions induced at the sliding interface, the total number of chemical bonds of both SiC substrate and SiC tip were analyzed (Figure 3 (a)~(b)). As shown in Figure 3 (a), it is interesting to observe that the number of Si-C and Si-Si bonds decreased, and however that of C-C bonds did not change, suggesting that C-C bonds hardly react with water molecules. As shown in Figure 3 (b), it is also interesting to see that the number of Si-O, Si-H, and C-H bonds increased, and however that of C-O bonds did not change, indicating that the C atoms hardly bonded with O atoms and selectively bonded with H atoms, although Si atoms preferentially bonded with O atoms. Figure 3 (c) shows that H2O molecules decreased during the friction simulations. These results indicate that the hydrolysis reactions, Si-C + H2O → Si-OH + C-H and Si-Si + H2O → Si-OH + Si-H occur at the friction interface, and however, C-C + H2O → C-OH + C-H hardly occurs. It means that the reactivity of C-C bonds with H2O molecules is significantly lower than those of Si-C and Si-Si bonds. Then we suggested that these selective chemical reactions of C atoms lead to the generation of hydrocarbon molecules and those of Si atoms lead to the generation of silicon oxide layers at the friction interface. Furthermore, to understand the structure of the generated oxide layer between the SiC substrate and SiC tip, we analyzed the number of Si-O-Si, Si-O-C, and C-O-C units. As shown in Figure 3 (d), the number of Si-O-Si units increased as friction progressed, while the number of Si-O-C and C-O-C units were almost constant. These results also indicate that Si atoms are preferentially oxidized and however C atoms are hardly oxidized, resulting in the generation of silicon oxide layers at the friction interface.

(a) Time evolution of the number of Si-C, Si-Si, and C-C bonds. (b) Time evolution of the number of Si-O, Si-H, C-H, and C-O bonds. (c) Time evolution of the number of H2O molecules. (d) Time evolution of the number of Si-O-Si, Si-O-C, and C-O-C units.
As shown in Figure 2 (200 ps and 300 ps), silicon oxide wear particles dissolved in water to form colloidal silica. In our previous simulation on the friction processes of SiO2 substrates [8]. We suggested that the colloidal silica layer is generated by the chemical reactions between SiO2 substrate and water molecules, preventing the contact of the surfaces and reducing the frictional forces. Similar to the SiO2/SiO2 friction in water environments, we concluded that at the SiC/SiC friction interface the friction-induced chemical reactions of water preferentially oxidizes the Si atoms in both the SiC substrates and SiC tips and then generates colloidal silica lubricating layers, preventing the contact of the surfaces and then reducing the frictional forces.
In the present study, reactive MD based sliding simulations of amorphous SiC in water environments were carried out. We found that the hydrolysis reactions of Si-C and Si-Si bonds occurred at the friction interface, however, those of C-C bonds hardly occurred. In the hydrolysis reactions of Si-C bonds, C atoms preferentially bonded with H atoms, and in the hydrolysis reactions of Si-C and Si-Si bonds, Si atoms preferentially bonded with O atoms. These selective chemical reactions generate hydrocarbon molecules and silicon oxides wear particles at the SiC/SiC friction interface. The silicon oxide wear particles dissolved in water leads to the formation of colloidal silica lubricant at the sliding interface, preventing the contact of the surfaces and reducing the frictional forces. Therefore, finally we concluded that the silicon oxide wear particles generated by the friction-induced chemical reactions are responsible for the super low friction of SiC.