2022 Volume 21 Issue 2 Pages 55-57
2022 Volume 21 Issue 2 Pages 55-57
π-conjugated carbon molecules such as fullerenes and carbon nanotubes have attracted great interest because of their potential applications as electronic materials and catalysts. Among these molecular frameworks, fullerene substructures of sumanene molecules with C3v symmetry are expected to form vertical columnar crystal structures and exhibit a range of conduction properties. Various syntheses of molecules with extended π-conjugation at the benzyl position of sumanene have now been reported. However, there are no examples of open-shell systems with extended sumanene features. In this study, we use the results of DFT calculations to discuss the relationship between molecular conformational symmetry and spin multiplicity in a new, extended open-shell system of sumanenes by adding a phenyl group at the benzyl position.
In recent years, organic π-conjugated compounds, the basic materials for practical applications in organic electronics such as organic transistors and organic EL, have attracted much attention in the field of novel molecular design and functional expression [1,2,3,4]. π-conjugated compounds exhibit optical absorption and luminescence properties due to the conjugation of π electrons and can conduct electricity by controlling intermolecular interactions and carrier generation. Since π-conjugated molecules generally have planar structures, new electronic materials based on molecules with nonplanar structures, such as fullerene C60 are expected to have unique electronic and physical properties derived from their 3D π-electron conjugated structures. Among them, the buckybowl-type compound sumanene (C21H12) [5], which corresponds to the partial structure of fullerenes, has the following characteristics: (i) the benzyl position is an sp3-hybridized carbon atom, which makes it easy to functionalize via radicals and cations; and (ii) in the crystalline state, it forms a column-like stacked structure derived from the bowl shape, and exhibits conductive properties [6, 7]. In this regard, sumanene is essential for creating new bowl-shaped compounds.
The column structure can be controlled by introducing various substituents at the benzyl position, and a range of extended sumanene derivatives have been reported to date [8]. However, there are no examples of open-shell systems in extended sumanene derivatives. In this study, we discuss the relationship between molecular conformational symmetry and spin multiplicity of a new sumanene derivative with a phenyl group at the benzyl position (triphenyl sumanene; see Figure 1) based on DFT calculations.

Structure of the triphenyl sumanene radical molecule.
Calculations were performed with the Gaussian 16. The DFT method was used to optimize the structure and to calculate the vibrational frequencies of triphenyl sumanene radical molecules at the B3LYP/6-31G (d,p) level. The B3LYP functional has been used extensively and has given the most reliable results for structure prediction of organic radical molecules [7].
The triphenyl sumanene radical molecule (Figure 1) considered in this study has triply degenerate SOMO localized at the three equivalent benzyl positions and has a quartet spin multiplicity, judging from the C3v symmetry structure of the original sumanene. However, steric hindrance between the phenyl groups causes the phenyl groups to twist and change orientation, thereby affecting the conformational symmetry and spin multiplicity of the radical molecule.
The optimized structure for the quartet state is shown in Figure 2. The sumanene structure was curved to ≈ 27°, yielding a structure with C1 symmetry (Figure 2 (a)), with one phenyl group twisted disrotatory (Tw-Dis), and a structure with C3 symmetry (Figure 2 (b)), with three phenyl groups twisted conrotatory (Tw-Con). The spin density distribution of Tw-Con (Figure 3) shows that the spins localize symmetrically at the three benzyl positions of sumanene. A comparison of the total energies (Table 1) revealed that Tw-Dis is slightly more stable by 0.005 eV and that the difference in the orientation of the twist of the phenyl group is small.
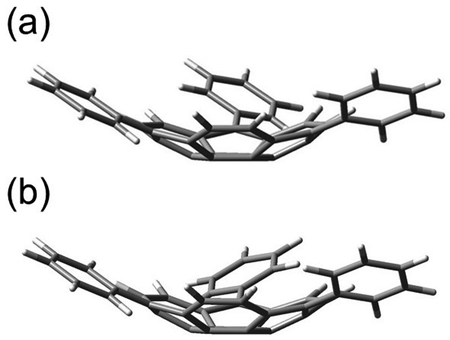
Optimized structures of phenyl sumanene molecules in the quartet state: (a) Tw-Dis (C1 symmetry), (b) Tw-Con (C3 symmetry).
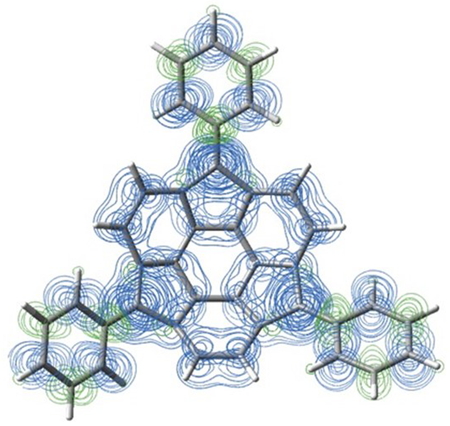
Spin density distribution of the quartet state Tw-Con.
| Doublet | Quartet | |||||
| Total Energy | Relative Energy | Relative Energy with ZPC | Total Energy | Relative Energy | Relative Energy with ZPC | |
| (Hartree) | (eV) | (eV) | (Hartree) | (eV) | (eV) | |
| Tw-Dis | −1498.7592 | −0.933 | −0.906 | −1498.7248 | 0.003 | −0.005 |
| Tw-Con | −1498.7577 | −0.893 | −0.873 | −1498.7249 | 0.000 | 0.000 |
The optimized structure for the doublet state, in which two of the three spins are singlet-coupled, is shown in Figure 4. As described for the quartet state, a structure with C1 symmetry, in which one phenyl group is twisted disrotatory (Tw-Dis, Figure 4 (a)), and a structure in which all phenyl groups are twisted conrotatory (Tw-Con, Figure 4 (b)), were obtained. The latter structure had C1 symmetry with slightly broken C3 symmetry. Both structures are curved by ≈ 30°, reflecting the increase in the sp3 nature of carbon atoms. Comparing the energies (see Table 1), we find that Tw-Dis is 0.040 eV (0.033eV with zero-point correction) more stable, as is the case for the quartet state, and that the difference in the orientation of the twist of the phenyl group has a more significant effect on the total energy for the doublet. The two unpaired electrons next to each other coupled antiferromagnetically rather than ferromagnetically in the ground state, resulting in the doublet energy being significantly lower than the quartet energy. The spin density distribution of Tw-Con shows (Figure 5) that a spin is localized in the benzyl position where the twist of the phenyl group is smaller than the others. This energy stabilization is due to a structural change involving symmetry breaking, i.e. the Jahn-Teller effect [9].
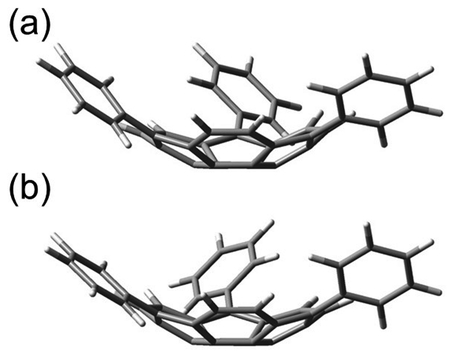
Optimized structures of triphenyl sumanene in the doublet state: (a) Tw-Dis (C1 symmetry), (b) Tw-Con (C3 symmetry).
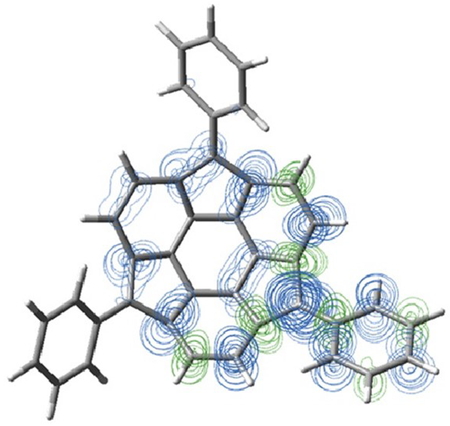
Spin density distribution of the doublet state Tw-Con.
In summary, the relationship between the symmetry of the molecular structure and the spin multiplicity of triphenyl sumanene radical molecule as a novel sumanene derivative radical molecule is discussed based on DFT calculations. It is found that the effect of the phenyl group on the total energy is more significant for the doublet state compared with the quartet state. It may be expected that it is easier to create a stable stacked structure for quartet multiplicity since pairs of stacking radical molecules can easily adjust their structures. We plan to study the structure symmetry and spin multiplicity of stacked systems of triphenyl sumanene radical molecules in the future.
We would like to thank Prof. K. Yamashita for fruitful discussions from experimental and theoretical perspectives. This work was supported by JSPS KAKENHI Grant Number 20A201. The computations were performed at the Research Center for Computational Science, Okazaki, Japan (Project: 22-IMS-C064) and the Center for Computational Materials Science, Institute for Materials Research, Tohoku University for the use of MASAMUNE-IMR (MAterials science Supercomputing system for Advanced MUlti-scale simulations toward NExt-generation-Institute for Materials Research).