2025 Volume 24 Issue 1 Pages 20-26
2025 Volume 24 Issue 1 Pages 20-26
Molecular dynamics (MD) simulations, accelerated by a universal neural network potential, were employed to investigate the dynamic behaviors of ten inorganic anions (Br-, Cl-, F-, OH-, NO3-, H2PO2-, H2PO3-, HPO42-, CO32- and SO42-) intercalated into NiFe layered double hydroxide at varying hydration levels. Our results show that the lattice parameters along the layered double hydroxide (LDH) layers are minimally affected by the intercalated anions and water content, while the lattice parameter perpendicular to the layers, i.e., the basal spacing, is strongly influenced by the type of anion and hydration level. The basal spacing is closely correlated with the ionic radius and charge of the anions, as well as the amount of water uptake. Mean squared displacement (MSD) analysis reveals distinct behaviors of the anions under different hydration conditions. While some anions, such as NO3- and H2PO2-, exhibit noticeable mobility, others remain largely immobilized, primarily due to strong electrostatic interactions with the LDH layers and water molecules. Hydration weakens the interaction between anions and the LDH but also restricts the mobility of anions due to the formation of hydration shells. These findings provide insights into anion dynamics and selectivity in NiFe LDH, which are critical for designing materials for anion removal applications.
Layered double hydroxides, with the formula [M2+1-xM3+x(OH)2]x+(An-)x/n·yH2O, consist of positively charged hydroxide layers where metal cations occupy octahedral sites, while charge-balancing anions and water reside in the interlayer galleries (Fig. 1a). This structural flexibility allows LDHs to host a wide range of anions, making them highly effective in applications such as photocatalysis, drug delivery, and environmental remediation [1]. Among the various LDH compounds, NiFe-based LDHs stand out for their ease of synthesis and tunable trivalent metal content or incorporation of other metals [2]. Importantly, NiFe LDHs exhibit excellent catalytic activity, particularly in oxygen evolution reactions, and possess outstanding anion-exchange capabilities [3, 4]. These features make them highly suitable for applications focused on the selective removal of toxic anions from aqueous environments. However, the detailed mechanisms governing anion interactions within their structure remain insufficiently understood. Gaining deeper insight into these interactions is essential for optimizing anion-exchange properties and enhancing their effectiveness in practical applications. For this, we employ molecular dynamics simulations accelerated by machine learning interatomic potentials to investigate how various anions interact when intercalated into the LDH. In this work, 10 commonly studied monovalent and divalent inorganic anions are selected. They are halides (Br-, Cl-, F-), hydroxide (OH-), nitrate (NO3-), hypophosphite (H2PO2-), phosphite (H2PO3-), phosphate (HPO42-), carbonate (CO32-) and sulfate (SO42-).

Figure (a) Schematic supercell structure of NiFe layered double hydroxide with space
group
We used a binary NiFe layered double hydroxide model with a Ni2+/Fe3+
ratio of 2:1 (x = 1/3). Total energy calculations were performed using a universal neural
network potential, namely the Preferred Potential (PFP) [5]. The choice of PFP in this study was motivated by its universal applicability
to diverse elements (supporting 96 naturally occurring elements from the periodic table) and
its ability to predict charge distributions, making it particularly well-suited for
simulating ionic systems such as the NiFe LDH investigated here. Furthermore, PFP has been
extensively tested in various applications, demonstrating its ability to achieve reasonable
accuracy across a wide range of material systems, including layered structures [6, 7],
multi-elemental nanoparticles and bulk structures [8,
9], gas molecule adsorption on nanoparticles [9, 10], and,
particularly, the dynamics of ionic systems [11]. In
fact, the suitability of PFP for modeling the NiFe LDH system was validated by comparing our
results, including basal spacing and X-ray Diffraction (XRD) patterns, with experimental
data. As shown in Table S1 and Fig. S1 in the Supplementary Information, good agreement was
found. In this work, PFP version 5.0.0 was used. PFP offers several calculation modes, which
support both molecular and crystal systems, with options to include or exclude Hubbard
corrections and dispersion corrections. For the case of NiFe, the mode for crystal systems
with Hubbard and dispersion corrections (CRYSTAL_PLUS_D3) was used to account for 3d
transition metals and layered structure. It is worth noting that in the calculations of
total energies using PFP with Hubbard correction, the dataset for training the potential was
prepared by applying the correction method by Dudarev et al. [12] with effective U values of 6.2 and 5.3 eV for Ni and
Fe, respectively. On the other hand, for the PFP mode including dispersion correction, the
DFT+D3 method by Grimme et al. [13,
14] was used for dealing with the dispersion
interaction. Initially, Ni and Fe were placed randomly on the cation sites of LDH layers.
The optimal atomic arrangement of Ni and Fe was then optimized using a simulated annealing
Monte Carlo method [6]. The results revealed a strong
preference for ordering, where Fe atoms tend to arrange in a
The calculated lattice parameters for the NiFe LDH cell along the

Basal spacing of NiFe LDH with the intercalation of various anions. Divalent anions are shown on the right-hand side and highlighted in the yellow area. The values of ionic radii are adapted from Refs. [19-22], except for phosphorus oxyanions, where the values (shown in red) are approximated based on their components and P–O and P–H bond lengths.
| Anion | An-:H2O = 1:0 | An-:H2O = 1:1 | An-:H2O = 1:2 | An-:H2O = 1:3 | ||||||||
| X | O | H | X | O | H | X | O | H | X | O | H | |
| Br- | -0.718 | -0.701 | -0.704 | -0.702 | ||||||||
| Cl- | -0.758 | -0.730 | -0.677 | -0.698 | ||||||||
| F- | -0.942 | -0.886 | -0.866 | -0.844 | ||||||||
| OH- | -1.323 | 0.626 | -1.332 | 0.672 | -1.315 | 0.677 | -1.303 | 0.661 | ||||
| NO3- | 0.831 | -0.619 | 0.825 | -0.601 | 0.827 | -0.585 | 0.816 | -0.577 | ||||
| H2PO2- | 3.069 | -1.625 | -0.362 | 3.092 | -1.617 | -0.347 | 3.087 | -1.620 | -0.336 | 3.097 | -1.620 | -0.330 |
| H2PO3- | 3.458 | -1.569 | 0.176 | 3.459 | -1.556 | 0.177 | 3.458 | -1.553 | 0.175 | 3.467 | -1.554 | 0.171 |
| CO32- | 1.989 | -1.211 | 1.969 | -1.210 | 1.984 | -1.206 | 1.995 | -1.208 | ||||
| SO42- | 3.757 | -1.418 | 3.790 | -1.407 | 3.819 | -1.395 | 3.820 | -1.387 | ||||
| HPO42- | 3.712 | -1.509 | 0.642 | 3.727 | -1.504 | 0.678 | 3.730 | -1.496 | 0.689 | 3.735 | -1.489 | 0.666 |
Fig. 3a presents mean-squared displacement for F- anions, with data shown for both anhydrous and hydrous states. We can see that in the anhydrous state, the MSD remained flat throughout the simulation, indicating that F− exhibited very limited mobility. This suggests that F- may be tightly bound to the LDH layers due to strong electrostatic interactions. In contrast, in the hydrous state, a gradual increase of MSD over time is observed. This implies that water weakens the interaction between F- and the hydroxide layers, allowing more free movement within the interlayer. Fig. 3b shows that in the anhydrous state, F- anions bind to the hydroxide groups of the LDH through hydrogen bonds (H-bonds) and are distributed evenly within the LDH interlayer. This arrangement, similar to that of trivalent cations (Fe3+), minimizes repulsion among the F- anions themselves. In the hydrous state, when water molecules are intercalated into the interlayer, the oxygen atoms in the water form H-bonds with the LDH hydroxide groups, weakening the interaction between the F- anions and the LDH. With a small amount of intercalated water, there is enough space between F- anions. Therefore, the basal spacing slightly expands. However, as more water is introduced, additional H-bonds form between water molecules and the LDH hydroxide groups, expanding the basal spacing quickly. Simultaneously, F- anions and water molecules form large hydration shells, further weakening the direct bonding between the F- anions and the LDH. For other halide anions (Br-, Cl-),a similar distribution and change in interlayer spacing were observed as in the case of F-. However, in the anhydrous state, Br- and Cl- can more easily jump between sites due to their weaker interaction with the LDH (see Fig. S2). When water is intercalated, the mobility of Br- and Cl- decreases because the formation of hydration shells creates a steric hindrance effect that restricts the movement of these anions.
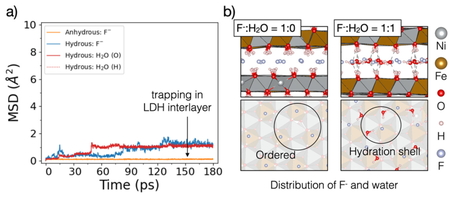
(a) MSD of fluoride (F-) and water in the anhydrous and hydrous conditions and (b) Distribution of fluoride and water in the LDH interlayer from the last MD snapshot. Ni2+ and Fe3+ cations are displayed as octahedra.
Fig. 4a shows that NO3- exhibits significantly higher mobility compared to halides. This suggests weaker interaction between NO3- and the LDH layers, allowing for more rapid diffusion. In the anhydrous state, NO3- exhibits linear increases in MSD, indicating diffusive behavior rather than jumping. In the hydrous state, the anion continued to show linear increases in MSD, but at a slower rate compared to the dehydrated state. The mobility of NO3- is reduced, likely due to the blocking of water that impedes diffusion. The diffusion coefficient dropped from 3.396×10−6 cm2/s to 1.261×10−6 cm2/s. Fig. 4b shows that unlike halides, the distribution of NO3- anions is not strictly even within the LDH interlayer. This might be due to the delocalized negative charge across the anion, as compared to F- or Cl-. In both anhydrous and hydrous states, NO3- anion prefers a perpendicular or slightly tilted orientation with the LDH layers. This orientation is similar to that observed from experiment for the case of CaAl LDH [23]. By comparing the interaction of NO3- and CO32- (which share similar atomic conformation) with LDH, we found that NO3- prefers a perpendicular or tilted orientation, while CO32- adopts a planar orientation within the LDH interlayer. Notably, this planar orientation of CO32- persists even as more water is introduced into the interlayer (see Fig. S3). The difference arises from their geometry and charge: NO3-, with its delocalized single negative charge (approximated from ionic radius and Bader charge in Fig. 2 and Table 1), exhibits flexibility, favoring perpendicular or tilted orientations due to weaker electrostatic interactions and versatile hydrogen bonding. In contrast, CO32-,with its higher charge density, strongly prefers an in-plane orientation, maximizing electrostatic attractions and hydrogen bonding with LDH layers.

(a) MSD of nitrate (NO3-) and water in the anhydrous and hydrous conditions and (b) Distribution of nitrate and water in the LDH interlayer from the last MD snapshot. Ni2+ and Fe3+ cations are displayed as octahedra, while NO3- anions are presented as triangles, with nitrogen (N) at the center.
For the remaining anions, we found that H2PO2- behaves similarly to NO3-, as it can diffuse within the LDH interlayer in the anhydrous state. However, when water is present, the anion is heavily blocked by water and becomes trapped in the LDH interlayer. From the estimation of the mean squared displacement of all anions, we found that, in the hydrous state with a hydration level of An−:H2O = 1:1, NiFe LDH has a high likelihood of selecting OH-, SO42-, HPO42-, CO32-, F-, H2PO3- from water, while the selection of NO3- and H2PO2- is less probable. This is qualitatively consistent with experimental observations [24]. In general, the observed differences in mobility between intercalated anions can be attributed to their hydration shell characteristics, steric hindrance (due to the presence of water), and competitive adsorption between the anions and water molecules. For instance, F- forms a strong hydration shell, which moves along with the water molecules during diffusion, allowing F- to exhibit increased mobility when water is introduced. In contrast, NO3- has a larger ionic radius and experiences steric hindrance in the presence of water, leading to reduced mobility. Additionally, for anions such as Br- and Cl-, which also show changes in mobility upon hydration, this can be explained by the competitive adsorption between the anions and water molecules, as well as their weaker hydration shells compared to F-. Experimentally, the hydration energies of Br-, Cl-, and F- are -104.0, -131.2, and -278.8 kJ/mol, respectively [25].
To further understand the interactions among anions, water, and LDH, dispersion interaction and short-range Coulomb interaction (modeled by the Yukawa potential) were analyzed. For the dispersion interaction, additional calculations were performed using the CRYSTAL mode of the potential and compared with data from the CRYSTAL_PLUS_D3 mode. We estimated that dispersion interactions contribute about 2.2% to the total energy, while Coulomb interactions between anions, water, and LDH (within a cutoff radius of 8 Å) account for approximately 7.0%. The primary contribution likely comes from mixed ionic-covalent interactions within the LDH layers and the hydrogen bonding network. Upon hydration, the dispersion energy slightly increases, while the Coulomb interaction energy rises more significantly. Further decomposition reveals that most of the Coulomb interaction comes from the interaction between anions and LDH hydroxide groups, as well as between anions and water. As the hydration level increases, the anion-LDH interaction weakens due to the formation of a hydration shell around the anions, which blocks direct interaction with the LDH hydroxide groups. Meanwhile, the interaction between anions and water increases (Fig. 5). Lastly, the hydrogen bonding network, particularly involving water molecules, may play a significant role in the mobility and interaction of intercalated anions with the LDH layers. Anions like F-, with a strong hydration shell, form robust hydrogen bonds with water molecules, facilitating their movement during diffusion. Similarly, Br- and Cl-, despite having weaker hydration shells compared to F-, also form hydrogen bonds with water, allowing for increased mobility upon hydration, albeit to a lesser extent. Although not discussed in detail here, this aspect warrants further investigation in future studies.

Short-range Coulomb interaction between the anion, LDH, and water at different hydration levels
We performed molecular dynamics simulations using a universal neural network potential to study the dynamic behaviors of ten inorganic anions (Br-, Cl-, F-, OH-, NO3-, H2PO2-, H2PO3-, HPO42-, CO32- and SO42-) intercalated into NiFe layered double hydroxide ([Ni2/3Fe1/3(OH)2]1/3+(An-)1/3n) at different hydration levels. First, we found that while the lattice parameters along the LDH layers are less sensitive to the intercalation of different anions and water levels, the lattice parameter perpendicular to the LDH layers is strongly influenced by the type of intercalated anions and the amount of water. The basal spacing of the LDH is closely correlated with the anion's ionic radius and charge state. Second, the mean squared displacement analysis reveals distinct behaviors for selected anions under varying hydration conditions, showing differences in mobility and interactions within the NiFe LDH structure. In the short-time MD simulations, the diffusive behavior of all anions, except NO3- and H2PO2-, was barely observed. These anions were mostly trapped in the interlayer due to their strong electrostatic interactions with the LDH hydroxide groups or surrounding water. Specifically, the presence of water significantly hindered the movement of the anions. Further analysis indicates that the majority of the Coulomb interactions stem from interactions between anions and the LDH hydroxide groups, as well as between anions and water. For future work, longer simulation times could be employed to better capture the anion selectivity and provide deeper insights into the stability and dynamics of intercalated anions over extended periods. Additionally, exploring the effects of varying ionic strengths and mixed-ion intercalation may further enhance the understanding of ion dynamics in LDH materials.
This work was supported by Council for Science, Technology and Innovation (CSTI), Cross-ministerial Strategic Innovation Promotion Program (SIP), the 3rd period of SIP "Creating a materials innovation ecosystem for industrialization".