Article ID: 2014-0009
Article ID: 2014-0009
After the accident of Fukushima's nuclear power plant, soil contamination was caused by radioactive cesium. In this study, the new cesium-adsorbent: Cs1-xNaxMnII(CN)3 Prussian blue analogue was theoretically designed to solve the problem. In Prussian blue (iron cyano-compound), both water defect and iron vacancy are closely related to cesium-adsorption. On the other hand, in Cs1-xNaxMnII(CN)3, the cesium-adsorption mechanism is completely different. Hybrid Kohn-Sham DFT calculations were performed to investigate the cesium-adsorption mechanism. It was concluded that more stable and efficient cesium-adsorption can be realized by the ion exchange between counter cations (between sodium and cesium ions), under applied voltage.
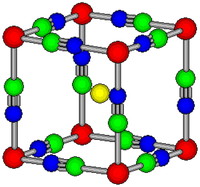
The cubic crystal structure of CsMnII(CN)3 at room temperature. The red, green, blue and yellow dots denote manganese, carbon, nitrogen and cesium, respectively.

The cluster models for Cs1-xNaxMnII(CN)3:(a) CsMnII8(CN)12, (b) NaMnII8(CN)12 models. Cesium or sodium ion migrates along x axis, following an arrow.

The schematic picture on Onishi chemical bonding rule.

The cesium-adsorption mechanism.

The potential energy curves for (a) CsMnII8(CN)12 and (b) NaMnII8(CN)12 models, displacing cesium and sodium ions along x axis (x = 0.0 ~ 2.65Å) in CsMnII8(CN)12 and NaMnII8(CN)12 models, respectively.

The molecular orbitals (MOs) related to the outer shell of sodium ion (2s and 2p orbitals) for NaMnII8(CN)12 model: (a) the cubic centre (x = 0.0Å), (b) minimum (x = 1.8Å), and (c) bottleneck (x = 2.65Å).

The selected molecular orbitals (MOs) related to the outer shell of cesium ion (5s and 5p orbitals) for CsMnII8(CN)12 model: (a) the cubic centre (x = 0.0Å), and (b) bottleneck(x = 2.65Å).

The schematic picture on the ion exchange between cesium and sodium ions in Cs1-xNaxMnII(CN)3, under applied voltage.