論文ID: 2015-0009
論文ID: 2015-0009
The nature of Si-O bonding and Si-O-Si bridging is discussed using molecular orbital calculations. We found the equilibrium geometries for two pyrosilisic acid molecules (C2V and 60° torsion) using Møller-Plesset perturbation theory and 6-311G (d,p) split valence basis set. The bent configuration of the Si-O-Si angle in equilibrium geometries can be explained by the balance of Coulombic repulsion between SiO4 tetrahedra and the energy of lone pair orbitals belonging to bridging oxygen atom without concerning the contribution of d-p π-bonding from the results of natural bonding orbital analysis. The energy surfaces of two pyrosilisic acid molecules with varying Si-O length to the bridging oxygen and Si-O-Si angle were calculated and we found the relationship between Si-O length to the bridging and Si-O-Si bridging angle. The Si-O bonding strengthens with increasing Si-O-Si angle because of stabilization in energy of Si-O bonding orbital with decreasing the hybridization index λ in spλ orbital of bridging oxygen and increase of Coulombic interaction between Si and bridging oxygen atom.

Calculated equilibrium geometries of pyrosilisic acid molecule. (a) C2V model. (b) Each tetrahedra mutually twisted 60° model.

The contour map of energy surface of pyrosilisic acid molecule (C2V) with varying Si-Obr and Si-O-Si angle.

The contour map of energy surface of pyrosilisic acid molecule (60° torsion) with varying Si-Obr and Si-O-Si angle.
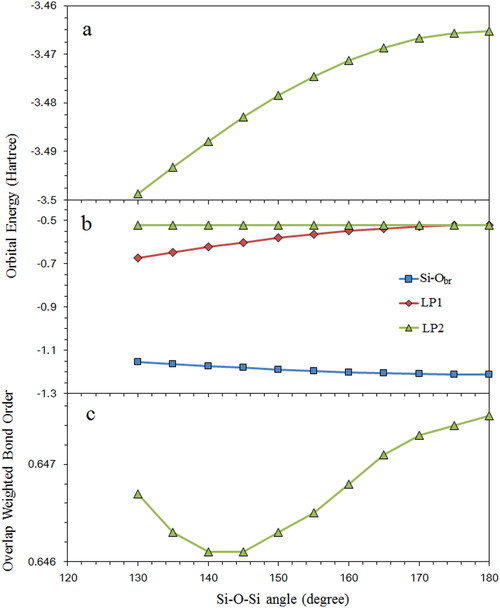
The plots of (a) sum of energy of the valence orbitals belongs to the bridging oxygen atom, (b) energy of each valence orbitals (two lone pairs (LP1, LP2) and Si-O bonding orbital (Si-Obr)) belongs to the bridging oxygen atom and (c) overlap weighted bond order of Si-Obr bonding with varying Si-O-Si angle in the case of 60° torsion model.

The plots of (a) electrostatic potential energy (ESP) between Si and bridging oxygen atoms calculated from charge and (b, c) charge of Si and bridging oxygen atoms calculated by NBO analysis with varying Si-O-Si angle in the case of 60° torsion. Si-Obr is the orbital energy of Si-Obr σ bond and plotted for comparison.

The plots of bonding coefficients Si and O atoms for Si-Obr σ-bonding with varying Si-O-Si angle in the case of 60° torsion.

The plots of (a) electrostatic potential energy of pyrosilisic acid molecule calculated from charge calculated by NBO analysis and (b) sum of the electrostatic potential energy and the energy of lone pair orbital with varying Si-O-Si angle.
| Exp. | HF | Δ | MP2 | Δ | B3LYP | Δ | PBE | Δ | |
| Si-O | 1.634 | 1.621 | −0.013 | 1.642 | 0.008 | 1.640 | 0.006 | 1.635 | 0.001 |
| Si-H | 1.486 | 1.476 | −0.010 | 1.476 | −0.010 | 1.485 | −0.001 | 1.488 | 0.002 |
| Si-O-Si | 144.1 | 180.0 | 35.9 | 156.5 | 12.0 | 179.2 | 35.1 | 169.7 | 25.6 |
| O-Si-H | 109.9 | 109.9 | 0.0 | 110.4 | 0.5 | 109.9 | 0.0 | 110.1 | 0.2 |
| H-Si-H | 109.1 | 109.0 | −0.1 | 109.0 | −0.1 | 109.0 | −0.1 | 109.0 | −0.1 |
| C2V | 60° torsion | |
| Si-Obr | 1.601171 | 1.604154 |
| Si-Onbr | 1.648782 | 1.648563 |
| O-H | 0.954669 | 0.954561 |
| Si-O-Si | 172.006 | 159.647 |
| Si-O-H | 117.705 | 117.857 |
| Total Energy (Hartree) | −1107.5476706 | −1107.5482842 |
| 130° | 155° | 180° | |
| 3s | 0.44388 | 0.43775 | 0.43546 |
| 3px + 3py + 3pz | 0.92376 | 0.91504 | 0.91303 |
| 3dxy + 3dyz + 3dzx + 3dx2y2 + 3d2 | 0.05959 | 0.05834 | 0.05808 |