Article ID: 2016-0049
Article ID: 2016-0049
八面体構造から四面体構造まで塩素数に応じて構造が変化する塩化鉄(III)錯体に対してDFT法を用いて理論計算を行った.使用した汎関数はBHandHLYP,wB97XD,CAM-B3LYP,LC-wPBEの4種である.基底関数には6-311+G(d,p)あるいは6-311+G(3df,2pd)を用い,PCM法による水媒体中で計算を行った.4種の汎関数を用いたDFT法は概ね錯体構造をよく再現した.2原子の塩素が配位した八面体型[Fe(III)Cl2(H2O)4]+のcis型とtrans型の安定性,及び3原子の塩素が配位した三方両錐型の[Fe(III)Cl3(H2O)2]と四面体型の[Fe(III)Cl3(H2O)] の安定性をエネルギー的に比較した.振動計算から得られる配位水分子の伸縮振動の波数の変化を[Fe(III)(H2O)6]3+から[Fe(III)Cl3(H2O)]まで調査したところ,八面体型では水分子の減少とともに伸縮振動は高波数側へシフトし,フリーの単独水分子の伸縮振動に近づく.八面体型では塩素原子の鉄への配位は鉄の極性を低下させ,その結果として水分子との相互作用を弱めると考えられる.しかし,三方両錐型([Fe(III)Cl3(H2O)2])から四面体型([Fe(III)Cl3(H2O)])になるにつれ再び伸縮振動はやや低波数側へシフトした.配位する塩素原子数が同じ3であっても,水分子数が少ないほど鉄との相互作用は強められる.鉄-酸素間距離についても同じ傾向を示した.
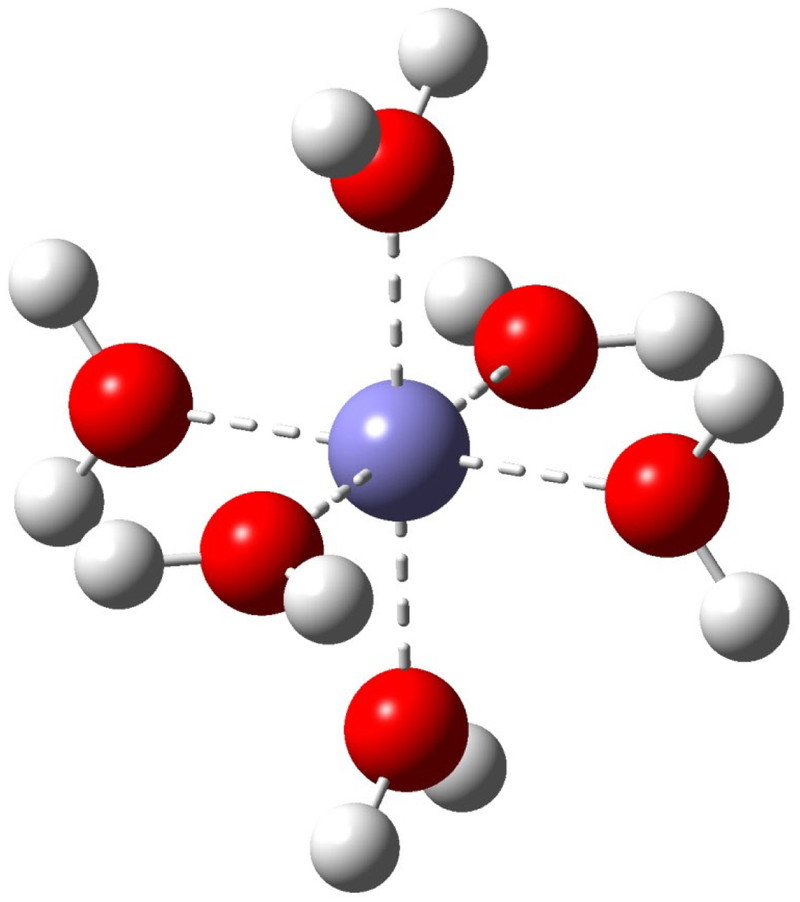
Optimized geometry of [Fe(III)(H2O)6]3+ with Th symmetry obtained by using wB97XD/6-311+G(3df,2pd) in PCM water.

Optimized geometry of [Fe(III)Cl(H2O)5]2+ with C2v symmetry obtained by using LC-wPBE/6-311+G(3df,2pd) in PCM water.

Optimized geometry of cis-[Fe(III)Cl2(H2O)5]+ with Cs symmetry obtained by using LC-wPBE/6-311+G(d,p). a) in PCM water and b) in vacuum.

Various optimized geometries of [Fe(III)Cl2(H2O)4]+ in PCM water obtained by using wB97XD/6-311+G(d,p). Point group symmetry and number of imaginary frequency are indicated at upper right of each geometry.

Optimized geometry of fac-[Fe(III)Cl3(H2O)3] and mer-[Fe(III)Cl3(H2O)3] obtained by using wB97XD/6-311+G(d,p) in PCM water.

Optimized geometry of trigonal bipyramidal [Fe(III)Cl3(H2O)2] and two types of tetrahedral [Fe(III)Cl3(H2O)] obtained by using LC-wPBE/6-311+G(d, p) in PCM water.

Relation of OH stretching frequency to number of H2O in various Fe(III) chloride complexes obtained by using wB97XD/6-311+G(3df, 2pd). T.B. means trigonal bipyramid. The OH stretching for free water is indicated as (○) and (●).

Relation of Fe − O distance to number of H2O in various Fe(III) chloride complexes obtained by using wB97Xd/6-311+G(3df, 2pd). T.B. means trigonal bipyramid.

Relation of ATP partial charge on Fe(III) to fraction of Cl in various Fe(III) chloride complexes obtained by using wB97Xd/6-311+G(3df, 2pd). T.B. means trigonal bipyramid.
| DFT method | P.G.M.S.a) | Im. Freq.b) | Fe—ODistance (pm) | Spin Densityon Fe | APT Chargeon Fe | APT Chargeon O | ν(OH)symν(OH)asym(cm−1) | |
| [Fe(III)(H2O)6]3+ | wB97XD/6-311+G(d, p) | Th | 0 | 201.2 | 4.394 | 3.144 | −0.917 | 3796.23880.3 |
| wB97XD/6-311+G(3df,2pd) | Th | 0 | 201.0 | 4.472 | 3.112 | −0.874 | 3807.53893.5 | |
| Exp. for[Fe(III)(H2O)6]3+ | — | — | 201c,d) 199e) | — | — | — | — | |
| [Fe(II)(H2O)6]2+ | wB97XD/6-311+G(d, p) | D2h | 8 | 211.0f)209.7217.9 | 3.845 | 2.064 | −0.774g)−0.817−0.775 | — |
| wB97XD/6-311+G(3df,2pd) | D2h | 9 | 210.8209.0217.6 | 3.864 | 2.043 | −0.737−0.778−0.738 | — |
a) Point group molecular symmetry
b) Number of imaginary frequency
c) Reference [8]
d) Reference [9]
e) Reference [10]
f) Due to the D2h symmetry, the three values are overlapped with each other among six kinds of Fe—O distance.
g) Due to the D2h symmetry, the three values are overlapped with each other among six kinds of charges on oxygen.
| DFT method | P.G.M.S.a) | Im. Freq.b) | Fe—ClDistance(pm) | Fe—ODistancec)(pm) | Spin Densityon Fe | APT Chargeon Fe | APT Chargeon Cl | APT Chargeon Od) |
| wB97XD/6-311+G(d, p) | C2v | 0 | 217.1 | 208.4204.4208.4 | 4.234 | 2.919 | −0.721 | −0.843−0.864−0.928 |
| wB97XD/6-311+G(3df, 2pd) | C2v | 0 | 216.6 | 208.3204.1210.9 | 4.337 | 2.904 | −0.722 | −0.813−0.826−0.896 |
| Exp. for[Fe(III)Cl(H2O)5]2+ | — | — | 228e)226f) | 200e) 205f) | — | — | — | — |
a) Point group molecular symmetry
b) Number of imaginary frequency
c) Due to the C2v symmetry, the first and the second values are overlapped among five kinds of Fe—O distance.
d) Due to the C2v symmetry, the first and the second charges are overlapped among five kinds of charges on oxygen.
e) Reference [4]
f) Reference [13]
| DFT method | P.G.M.S.a) | Im.Freq.b) | Fe—ClDistance(pm) | Fe—ODistance(pm) | Spin Densityon Fe | APT Chargeon Fe | APT Chargeon Cl | APT Chargeon O |
| cis-[Fe(III)Cl2(H2O)4]+ | ||||||||
| wB97XD/ 6-311+G(d, p) | C1 | 0 | 222.9222.1 | 210.4212.8219.1210.2 | 4.157 | 2.950 | −0.875−0.859 | −0.835−0.888−0.848−0.836 |
| wB97XD/ 6-311+G(3df,2pd) | C1 | 0 | 222.2221.5 | 210.6213.2219.4210.0 | 4.264 | 2.921 | −0.865−0.852 | −0.804−0.853−0.830−0.803 |
a) Point group molecular symmetry
b) Number of imaginary frequency
| DFT method | P.G.M.S. a) | Im.Freq.b) | Fe—ClDistance(pm) | Fe—ODistance(pm) | Spin Densityon Fe | APT Chargeon Fe | APT Chargeon Cl | APT Chargeon O |
| trans-[Fe(III)Cl2(H2O)6]+ | ||||||||
| wB97XD/ 6-311+G(d, p) | D2h | 4 | 227.1 | 210.7206.4 | 4.196 | 3.265 | −1.027 | −0.852−0.862 |
| D4h | 4 | 225.5 | 208.8 | 4.185 | 3.205 | −1.000 | −0.845 | |
| C2v | 2 | 226.6 | 208.6209.0 | 4.192 | 3.191 | −0.994 | −0.862−0.845 | |
| C1 | 0 | 225.9226.7 | 211.2208.9209.1208.8 | 4.189 | 3.169 | −0.990−0.998 | −0.828−0.847−0.840−0.844 | |
| wB97XD/ 6-311+G(3df, 2pd) | C1 | 0 | 225.3226.2 | 211.4208.8209.0208.6 | 4.196 | 3.129 | −0.982−0.995 | −0.801−0.814−0.809−0.812 |
| Exp. for[Fe(III)Cl2(H2O)6]+ | — | — | 230c) 229d) | 204c)208d) | — | — | — | — |
a) Point group molecular symmetry
b) Number of imaginary frequency
c) Reference [4]
d) Reference [19]
| DFT method | Conformer | P.G.M.S.a) | Cl-Fe-Cl(o) | Relative Energyb)(kJ/mol) |
| in water | ||||
| wB97XD/ 6-311+G(d, p) | cis | C1 | 104.19 | −11.24 |
| trans | C1 | 173.52 | 0 | |
| wB97XD/ 6-311+G(3df,2pd) | cis | C1 | 104.27 | −9.84 |
| trans | C1 | 173.09 | 0 | |
| in vacuum | ||||
| wB97XD/ 6-311+G(d,p) | cis | C1 | 108.83 | +9.68 |
| trans | C1 | 180.0 | 0 | |
| wB97XD/ 6-311+G(3df,2pd) | cis | C1 | 108.82 | +10.32 |
| trans | C1 | 180.0 | 0 |
a) Point group molecular symmetry
b) Values are obtained as the energy of the trans form is assumed to be zero
| DFT method | P.G.M.S.a) | Im. Freq.b) | Fe—ClDistance(pm) | Fe—ODistance(pm) | Spin Densityon Fe | APT Chargeon Fe | APT Chargeon Cl | APT Chargeon O |
| fac-[Fe(III)Cl3(H2O)3] | ||||||||
| wB97XD/ 6-311+G(d, p) | C1 | 0 | 226.5226.3226.4 | 220.4220.7220.8 | 4.076 | 2.983 | −0.925−0.921−0.920 | −0.846−0.844−0.846 |
| wB97XD/ 6-311+G(3df,2pd) | C1 | 0 | 225.9225.9225.9 | 220.7220.5221.3 | 4.186 | 2.959 | −0.916−0.914−0.913 | −0.829−0.829−0.822 |
| mer-[Fe(III)Cl3(H2O)3] | ||||||||
| wB97XD/ 6-311+G(d, p) | C1 | 0 | 230.9230.8227.0 | 220.2214.0212.4 | 4.133 | 3.145 | −1.004−1.005−0.931 | −0.843−0.834−0.835 |
| wB97XD/ 6-311+G(3df,2pd) | C1 | 0 | 230.3230.3226.2 | 221.0214.0212.3 | 4.231 | 3.106 | −1.009−1.008−0.922 | −0.825−0.809−0.805 |
a) Point group molecular symmetry
b) Number of imaginary frequency
| DFT method | P.G.M.S. a) | Im. Freq.b) | Fe—ClDistance(pm) | Fe—ODistance(pm) | O − Fe − OAngle(o) | Spin Densityon Fe | APT Chargeon Fe | APT Chargeon Cl | APT Chargeon O |
| wB97XD/6-311+G(d, p) | C1 | 0 | 224.3221.5225.4 | 213.6214.0 | 169.23 | 4.105 | 2.825 | −0.925−0.858−0.925 | −0.836−0.826 |
| wB97XD/6-311+G(3df, 2pd) | C1 | 0 | 223.7220.6224.6 | 213.8214.5 | 168.85 | 4.226 | 2.775 | −0.919−0.844−0.917 | −0.801 |
a) Point group molecular symmetry
b) Number of imaginary frequency
| DFT method | P.G.M.S.a) | Im. Freq.b) | Fe—ClDistance(pm) | Fe—ODistance(pm) | Cl − Fe − ClAngle (o) | Spin Densityon Fe | APT Chargeon Fe | APTChargeon Cl | APT Chargeon O |
| wB97XD/6-311+G(d, p) Type A | C1 | 0 | 219.3219.4219.9 | 205.2 | 115.0113.6114.1 | 4.056 | 2.643 | −0.876−0.883−0.882 | −0.837 |
| Type B | C1 | 0 | 219.4219.7220.1 | 205.1 | 114.9114.8113.5 | 4.056 | 2.630 | −0.874−0.868−0.881 | −0.840 |
| wB97XD/6-311+G(3df, 2pd) Type A | C1 | 0 | 218.8218.8218.9 | 205.3 | 115.0113.6114.1 | 4.150 | 2.577 | −0.858−0.863−0.859 | −0.793 |
| Type B | C1 | 0 | 219.0219.1219.2 | 205.1 | 115.0113.6114.1 | 4.150 | 2.565 | −0.854−0.850−0.859 | −0.795 |
a) Point group molecular symmetry
b) Number of imaginary frequency
| DFT method | BSSE corrected complexation energy(kJ/mol) | νsym(OH)(cm−1) | νasym(OH)(cm−1) | ω(H−O−H)(cm−1) |
| [Fe(III)Cl3(H2O)2] | ||||
| wB97XD/6-311+G(3df, 2pd) | −186.7a) | 3832.8(0.986)b)3833.5(0.986) | 3923.4(0.986)b)3923.6(0.986) | 1615.7(0.996)b)1624.3(1.001) |
| [Fe(III)Cl3(H2O)] | ||||
| wB97XD/6-311+G(3df, 2pd) | −132.2c) | 3826.5(0.984) | 3911.9(0.983) | 1637.3(1.010) |
| H2O in PCM water | ||||
| wB97XD/6-311+G(3df, 2pd) | — | 3887.3 | 3978.5 | 1621.9 |
a) complexation energy according to the equation: 2H2O + [Fe(III)Cl3] → [Fe(III)Cl3(H2O)2]
b) ratio to the respective wave number of H2O in PCM water
c) complexation energy according to the equation: H2O + [Fe(III)Cl3] → [Fe(III)Cl3(H2O)]
| DFT method | P.G.M.S.a) | Im. Freq.b) | Fe—ClDistance(pm) | Spin Densityon Fe | APT Chargeon Fe | APT Chargeon Cl | ν3(Fe-Cl) (cm−1) | ν4(Cl-Fe-Cl) (cm−1) |
| wB97XD/6-311+G(d) | Td | 2 | 223.2 | 4.081 | 2.642 | −0.910 | 384.3 | — |
| wB97XD/6-311+G(3df) | Td | 0 | 222.4 | 3.993 | 2.561 | −0.890 | 386.5 | 101.4 |
| Exp. | — | — | 220.4c) | — | — | — | 379d)376e) | 137d)135e) |
a) Point group molecular symmetry
b) Number of imaginary frequency
c) Cambridge Structural Database version 5.25, November 2003.
d) Reference [23].
e) Reference [24]