Article ID: 2017-0069
Article ID: 2017-0069
最近のMOPAC2016ハミルトニアンPM7を用いて, 基礎的な化学結合, 水素結合(Hb), 立体選択的Diels-Alder (DA) 反応, 実用的なイソシアネートのウレタン化反応そして光異性化反応等のシミュレーション解析を行い, その構造とエネルギー情報につき、実験値および他の計算値と比較評価した. 上記一部につき前報[1]では PM6法で可成りの理解は得られたが, 問題点のあることも指摘した. PM7での解析では、分子内、分子間の結合 距離とエネルギーの小さい変化の評価で向上が見られ, 分散力算出等の改善の有効性が示唆された. 例えば上記 反応2例では、より大きい水素結合エネルギー(EHb)と分子間相互作用エネルギー(ELn)の算出で安定錯体 (B)の存在推定と, より実験値に近い活性化エネルギー(Ea)が見積られ, Figure 1等の反応プロセスと遷移状態(TS)の評価改善が推定され, またBなどの低温での解析実験法を提案した. 簡便で定量性の改善された MOPAC2016は教育や実験の現場等で積極的活用が期待される.

PM7simulations of water dimer and methanol dimer structures (Å).

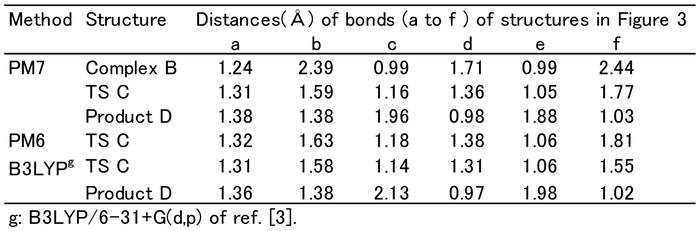
PM7 simulation of the (PhNCO + 2MeOH) reaction: The complex (B), transition state (TS: C), product (D) and relative energies (ΔHf). (Each Hf energy (kcal mol−1)). (subscript: Hb; Hydrogen bond, In; Interaction and a; activation).

IRC data of urethane (D) formation via the complex (B) from the (PhNCO + 2MeOH) reaction.

PM7-TS simulation of DA reaction (BD + ET), and the complex (B), transition state (TS: C) and product (D) energies: relative energies ΔHf (kcal mol−1).

Stereoselective (CP + MA) DA reaction processes, and the two complexes (Bend, Bexo), transition states (TSend, TSexo), and products (Dend, Dexo).

Valence isomerization of substituted dithienylethenes (VIo.Ph, VIc.Ph) and (VIo.CHO, VIc.CHO).