論文ID: 2021-0028
論文ID: 2021-0028
Nanoparticles have a wide range of applications as catalysts. Their catalytic and electronic properties differ from those of materials with flat surfaces and bulk materials. First-principles calculations of real system nanoparticles, which use nanoparticle models based on real shapes extracted from experimental observations, are essential for studying these properties to facilitate the computational design of new catalysts. In this article, we review first-principles studies of models of real systems of monometallic, bimetallic, and supported nanoparticles. The stability, electronic structure, hydrogen absorption behavior, and small molecule adsorption behavior are reviewed, and advances in first-principles calculations of real system nanoparticles are presented. Further, a combination of machine learning and first-principles studies is also considered. Future perspectives are discussed on the basis of these examples.

Size dependence of cohesive energy of Ru nanoparticles. Red squares, blue diamonds, magenta crosses, and green circles represent fivefold twinned decahedral fcc, icosahedral fcc, truncated octahedral fcc, and hcp structures, respectively. The broken lines represent regression lines. The inset shows an enlarged view at N−1/3 = 0.18−0.24. Note that N represents the number of atoms. [Reprinted with permission from Yusuke Nanba, Takayoshi Ishimoto, Michihisa Koyama. Structural Stability of Ruthenium Nanoparticles: A Density Functional Theory Study. The Journal of Physical Chemistry C 2017, 121 (49), 27445–27452. DOI: 10.1021/acs.jpcc.7b08672. Copyright 2017 American Chemical Society.]

Twin boundary models for fcc {111} stacking ∑3. The original fcc {111} stacking model ∑1 is arranged in ABCABC order. [Reprinted with permission from Yusuke Nanba, Takayoshi Ishimoto, Michihisa Koyama. Structural Stability of Ruthenium Nanoparticles: A Density Functional Theory Study. The Journal of Physical Chemistry C 2017, 121 (49), 27445–27452. DOI: 10.1021/acs.jpcc.7b08672. Copyright 2017 American Chemical Society.]

Side view (top two rows) and cross-sectional view (bottom two rows) of core–shell and SS nanoparticles of Pd201Pt510, Pt405Pd306, Pd405Pt306, and Pt201Pd510 after geometry optimization. Green and gray balls represent Pd and Pt atoms, respectively. [Reprinted with permission from Takayoshi Ishimoto, Michihisa Koyama. Electronic Structure and Phase Stability of PdPt Nanoparticles. The Journal of Physical Chemistry Letters 2016 7 (5), 736–740. DOI: 10.1021/acs.jpclett.5b02753. Copyright 2016 American Chemical Society.]

Excess energy of SS and core–shell nanoparticles of Pd201Pt510, Pt405Pd306, Pd405Pt306, and Pt201Pd510 from Pd711 and Pt711 nanoparticles (a) without entropy and (b) with entropy correction at 373 K. Calculated values for bulk PdPt SS systems (Pd0.25Pt0.75, Pd0.5Pt0.5, and Pd0.75Pt0.25) are shown as a reference. Cross-sectional views of core–shell nanoparticles are shown. Green and gray balls represent Pd and Pt atoms, respectively. [Reprinted with permission from Takayoshi Ishimoto, Michihisa Koyama. Electronic Structure and Phase Stability of PdPt Nanoparticles. The Journal of Physical Chemistry Letters 2016 7 (5), 736–740. DOI: 10.1021/acs.jpclett.5b02753. Copyright 2016 American Chemical Society.]

Calculated excess free energy and configurational entropy for different configurations of Pt3M nanoparticles (M = Co, Ni, and Cu), where SS, S1, and S2 refer to the SS configuration, a one-skin layer configuration, and a two-skin layer configuration, respectively. [Reprinted with permission from David S. Rivera Rocabado, Yusuke Nanba, Michihisa Koyama. Electronic Structure and Phase Stability of Pt3M (M = Co, Ni, and Cu) Bimetallic Nanoparticles. Computational Material Science 2020 184, 109874-1-9. DOI: 10.1016/j.commatsci.2020.109874. Copyright 2020 Elsevier.]

Calculated vs. predicted excess energy of Pt3M nanoparticles with S1 and S2 configurations. [Reprinted with permission from David S. Rivera Rocabado, Yusuke Nanba, Michihisa Koyama. Electronic Structure and Phase Stability of Pt3M (M = Co, Ni, and Cu) Bimetallic Nanoparticles. Computational Material Science 2020 184, 109874-1-9. DOI: 10.1016/j.commatsci.2020.109874. Copyright 2020 Elsevier.]

Results of first-principles calculations of d-band centers in
different Ru nanoparticle structures: (a) decahedral fcc, (b) icosahedral fcc, (c)
truncated octahedral fcc, and (d) hcp) and for different numbers of atoms (given as

DOS of Pt nanoparticles. (a) Partial DOS, (b) difference in partial DOS between contiguous nanoparticles, and (c) partial DOS of atoms in the outer shell of Pt nanoparticles. [Reprinted with permission from David S. Rivera Rocabado, Takayoshi Ishimoto, Michihisa Koyama. The Effect of SnO2(110) Supports on the Geometrical and Electronic Properties of Platinum Nanoparticles. SN Applied Sciences 2019 1, 1485. DOI: 10.1007/s42452-019-1478-0. Copyright 2019 Springer. CC by 4.0.]

(a) Size dependence of d-band center of Pt nanoparticles (gray circles) and of the d-band center of only the outer-shell atoms (blue squares). The values for bulk Pt (dashed purple line) and Pt (111) (dashed orange line) are shown for reference. (b) Relationship between the d-band center and the charge per atom of the outer-shell atoms of the nanoparticles. The linear regression line (dashed black line) and coefficient of determination are also shown. [Reprinted with permission from David S. Rivera Rocabado, Takayoshi Ishimoto, Michihisa Koyama. The Effect of SnO2(110) Supports on the Geometrical and Electronic Properties of Platinum Nanoparticles. SN Applied Sciences 2019 1, 1485. DOI: 10.1007/s42452-019-1478-0. Copyright 2019 Springer. CC by 4.0.]

Calculated d-band centers from total and partial DOS in core–shell and SS PdPt nanoparticles. [Reprinted with permission from Takayoshi Ishimoto, Michihisa Koyama. Electronic Structure and Phase Stability of PdPt Nanoparticles. The Journal of Physical Chemistry Letters 2016 7 (5), 736–740. DOI: 10.1021/acs.jpclett.5b02753. Copyright 2016 American Chemical Society.]

Calculated average atomic charge of each layer in SS and core–shell Pd201Pt510, Pt405Pd306, Pd405Pt306, and Pt201Pd510 nanoparticles. Pd and Pt appear as gray and green, respectively in the cross-sectional view of each nanoparticle. [Reprinted with permission from Takayoshi Ishimoto, Michihisa Koyama. Electronic Structure and Phase Stability of PdPt Nanoparticles. The Journal of Physical Chemistry Letters 2016 7 (5), 736–740. DOI: 10.1021/acs.jpclett.5b02753. Copyright 2016 American Chemical Society.]

Calculated d-band centers of surface Pt atoms in monometallic Pt nanoparticles and in S1 (diamonds), S2 (crosses), and SS (circles) Pt3M nanoparticles. [Reprinted with permission from David S. Rivera Rocabado, Yusuke Nanba, Michihisa Koyama. Electronic Structure and Phase Stability of Pt3M (M = Co, Ni, and Cu) Bimetallic Nanoparticles. Computational Material Science 2020 184, 109874-1-9. DOI: 10.1016/j.commatsci.2020.109874. Copyright 2020 Elsevier.)

Models of supported Pt nanoparticles. [Reprinted with permission from David S. Rivera Rocabado, Takayoshi Ishimoto, Michihisa Koyama. The Effect of SnO2(110) Supports on the Geometrical and Electronic Properties of Platinum Nanoparticles. SN Applied Sciences 2019 1, 1485. DOI: 10.1007/s42452-019-1478-0. Copyright 2019 Springer. CC by 4.0.]

Top: charge distribution of Pt atoms within nanoparticles. Bottom: average charge per atom for the outer-shell atoms in different layers within isolated Pt nanoparticles and SnO2-supported Pt nanoparticles. [Reprinted with permission from David S. Rivera Rocabado, Takayoshi Ishimoto, Michihisa Koyama. The Effect of SnO2(110) Supports on the Geometrical and Electronic Properties of Platinum Nanoparticles. SN Applied Sciences 2019 1, 1485. DOI: 10.1007/s42452-019-1478-0. Copyright 2019 Springer. CC by 4.0.]

(a) Charge variation for a supported single Pt atom and Pt atoms in a supported Pt4 nanoparticle on different support materials (SnO2, SnO2-δ, and Sn0.98Nb0.02O2). (b) Average charges of outer-shell Pt atoms in SnO2-supported Pt37 nanoparticle, graphene-supported Pt37 nanoparticle, and isolated Pt37 nanoparticle. [Reprinted with permission from David S. Rivera Rocabado, Takayoshi Ishimoto, Michihisa Koyama. The Effect of SnO2(110) Supports on the Geometrical and Electronic Properties of Platinum Nanoparticles. SN Applied Sciences 2019 1, 1485. DOI: 10.1007/s42452-019-1478-0. Copyright 2019 Springer. CC by 4.0.]
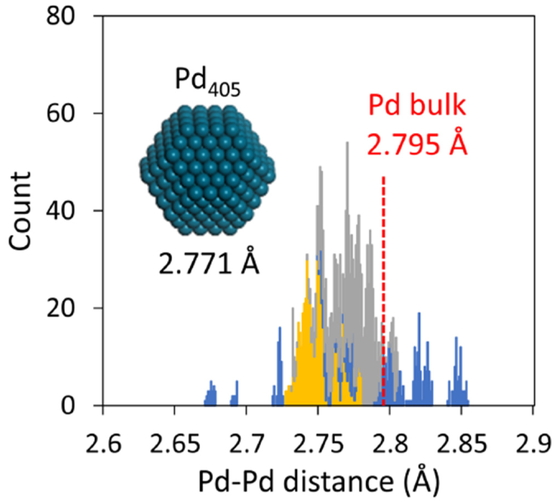
Distribution of nearest-neighbor interatomic distance in a real Pd405 model (yellow: distribution on the surface, blue: distribution in the interlayer between surface and subsurface layers). (Reproduced with the permission of AIP Publishing from Takayoshi Ishimoto, Michihisa Koyama. Theoretical Study of Tetrahedral Site Occupation by Hydrogen in Pd Nanoparticles. The Journal of Chemical Physics 2018 148, 034705. DOI: 10.1063/1.5005976.)

Absorption energy of hydrogen on (a) octahedral and (b) tetrahedral sites in a Pd405 nanoparticle with and without ZPE correction. (Reproduced with the permission of AIP Publishing from Takayoshi Ishimoto, Michihisa Koyama. Theoretical Study of Tetrahedral Site Occupation by Hydrogen in Pd Nanoparticles. The Journal of Chemical Physics 2018 148, 034705. DOI: 10.1063/1.5005976.)

Hydrogen diffusion mechanism in core and subsurface regions in Pd nanoparticles. (Reproduced with the permission of AIP Publishing from Takayoshi Ishimoto, Michihisa Koyama. Theoretical Study of Tetrahedral Site Occupation by Hydrogen in Pd Nanoparticles. The Journal of Chemical Physics 2018 148, 034705. DOI: 10.1063/1.5005976.)

(a) Top, (b) bridge, and (c) hollow adsorption sites on M405 nanoparticles. The (100) and (111) facets are represented by red squares and green hexagons, respectively. Orange symbols: adsorption sites on ridge (T1x, B1x); red symbols: adsorption sites on (100) facet (T2x, B2x, H2x); green symbols: adsorption sites on (100) facet (T2x, B2x, H2x). [Reprinted with permission from Yusuke Nanba, Michihisa Koyama. NO Adsorption on 4d and 5d Transition-Metal (Rh, Pd, Ag, Ir, and Pt) Nanoparticles: Density Functional Theory Study and Supervised Learning. The Journal of Physical Chemistry C 2019 123 (46), 28114–28122. DOI: 10.1021/acs.jpcc.9b05748). Copyright 2019 American Chemical Society.]
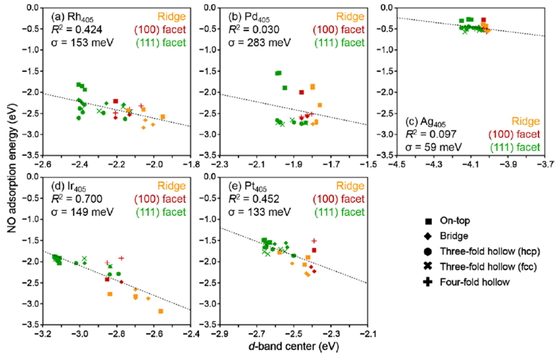
NO adsorption energy at different sites for different facets of (a) Rh405,
(b) Pd405, (c) Ag405, (d) Ir405, and (e)
Pt405 nanoparticles. The coefficient of determination

Comparison of NO adsorption energies predicted by multiple regression analysis (SL)
and those calculated by DFT for M405 nanoparticles, where M = (a) Rh, (b) Pd,
(c) Ag, (d) Ir, and (e) Pt.