2017 Volume 66 Issue 1 Pages 13-19
2017 Volume 66 Issue 1 Pages 13-19
In this review, we demonstrate that the practical applications of a novel long-chain amidoamine derivative with a simple molecular structure (C18AA) are equivalent to or higher in number than those of supramolecules with complex molecular structures. Molecular assemblies of C18AA exhibit distinctive thermal responsiveness; namely, C18AA can form a gel with apolar organic solvents, while O/W emulsions of C18AA act as a heat-induced gelator that undergoes a phase transition from solution to gel upon heating. We also show that C18AA emulsions containing a quaternary ammonium salt develop an iridescent color over a specific temperature range. Further, molecular assemblies of C18AA serve as high-performance soft templates for the preparation of shape-controlled metal nanocrystals such as ultrathin Au nanowires and Pd nanowires.
Nanoscale objects have attracted considerable interest on account of their unique properties and potential applications in the various fields of material science1),2). The construction and organization of nanoscale objects with desired components and shape are essential for fabricating novel functional materials, and one of the key concept in constructing and organizing the nanoscale objects is molecular self-assembly. Molecular self-assembly is a very common phenomenon of long-chain amphiphilic compounds in water or organic solvents3),4),5). The types and morphologies of the assemblies produced using conventional amphiphiles can be tuned by altering the amphiphle's concentration or the external conditions such as temperature or pH, although the variety of morphologies observed tends to be limited. Furthermore, it is generally difficult to obtain the assemblies with complicated morphologies using conventional amphiphiles, because the molecular interactions between the amphiphiles are mainly nondirectional. On the other hand, the rational introduction of functional moieties with the ability to take part in directional interactions with amphiphiles can generate the so-called supramolecules which can form more complex structures such as nanotubes or nanoribbons6),7). These complex structures can be changed reversibly by varying the external conditions, because the directional interactions between the amphiphiles are relatively weak, such as non-covalent bonding interactions such as hydrogen bonding interaction and π-π stacking interaction.
Although supramolecules are functionally superior to conventional amphiphiles, their synthesis generally requires many reaction steps due to their complex molecular structures. Thus, if we demonstrae that amphiphiles with simple molecular structures can assemble into various assemblies with the desired practical functions, it would open the door to new applications for such molecular assemblies.
Recently, we synthesized an amphiphilic long-chain amidoamine derivative(N-(2-amino-ethyl)-3-{[2-(2-amino-ethylcarbamoyl)-ethyl]-octadecyl-amino}-propioamide(C18AA; Fig. 1)with a simple molecular structure. Here, in order to impart this derivative with the ability to assemble into higher-order molecular assemblies mediated through hydrogen bonding interactions, we selected amide and amine groups as the key moieties to drive the directional and non-covalent bonding interactions. In this review, we demonstrate the multifunctional nature of C18AA and, in particular, its ability to act as a stimuli-responsive material and a superior soft-template for the preparation of shape-controlled metal nanocrystals8),9),10),11),12),13),14),15),16),17),18),19),20),21),22).
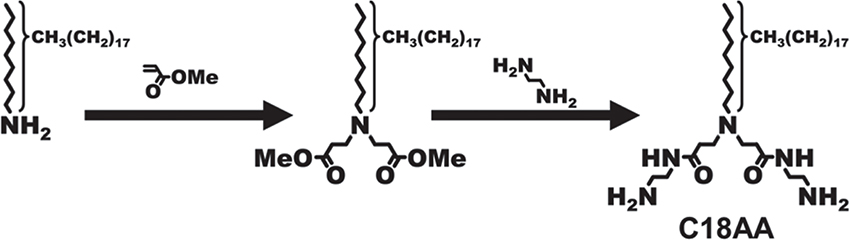
Molecular structure of C18AA and the synthesis approach.
C18AA shows robust gelation capacity in apolar solvents such as toluene, benzene, and cyclohexane9),13). The rigidity of organogels formed from C18AA is equal to that observed for typical low-molecular-weight gelators. Sol-gel transition temperatures(Tgel)of C18AA organogels is ~45°C, regardless of the organic solvents employed and the concentration of C18AA. The complex viscosity profiles of C18AA organogels showed that the phase transition from sol to gel occurs at 45°C. SEM images of C18AA xerogels revealed that the morphology of C18AA in the gel adopts three-dimensional flake structures, reminiscent of carnation flower petals. Further, the appearance of the periodical peaks in the XRD pattern of the organogel revealed that C18AA aggregates have lamellar structures in the gels. In addition, the lamella is considered to consist of a bilayer structure of C18AA, because head-to-head and tail-to-tail arrangements are favorable for amphiphilic molecules. Furthermore, FT-IR spectra for the toluene-d8 gel and CDCl3 solution of C18AA were measured. Observed bands at 3445, 1658, and 1518 cm-1 in the CDCl3 solution were assigned to the amide A and amide I and II, respectively. These bands appeared at 3330, 1644, 1543 cm-1 in the spectrum of the toluene gel. Since the amide A, and amide I and II bands for non hydrogen-bond(free)states are expected to be observed at ~3430, ~1650 and ~1510 cm-1, respectively, the appearance at lower frequencies for amide A and amide I, and the higher frequency of amide II in the gel spectrum, indicates that the amide groups are linked through hydrogen bonding to adjacent amide groups. Thus, the network of the self-assembled C18AA aggregates is mainly formed through hydrogen-bonding interactions. Fig. 2 shows the proposed lamellar structure of C18AA aggregates in the organogels, where the hydrocarbon chains are interdigitated and the adjacent amide groups are interacting through hydrogen bonding8),9).
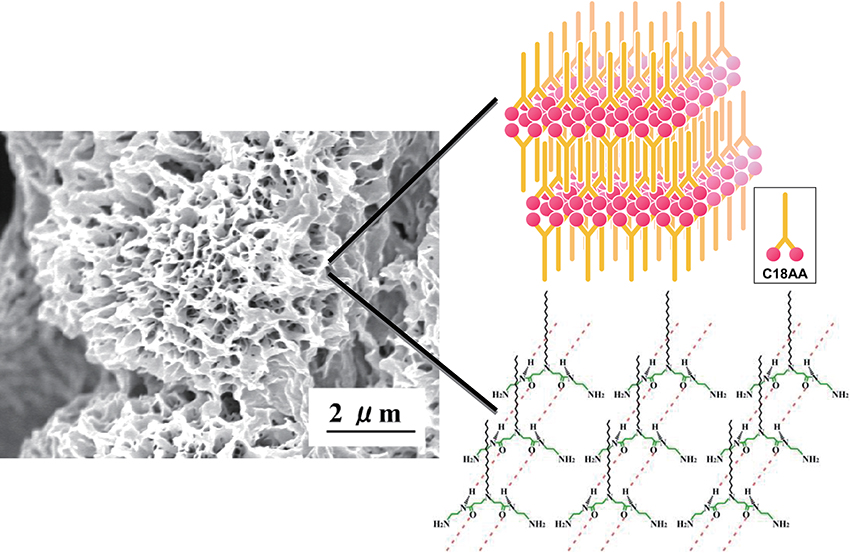
SEM image of C18AA xerogel made from an organogel and schematic illustration of a proposed hydrogen-bonding network and a lamellar structure of C18AA in the organogel.
Normal organogels fuse at high temperatures, whereas heat-induced gels undergo a thermally reversible phase transition from a solution to a rigid gel on heating. Heat-induced gels have attracted considerable attention as injectable drug-delivery systems; however, the number of such reports is limited. Interestingly, an opaque emulsion of C18AA shows a heat-induced gelation property, namely, it forms a rigid gel state at higher temperatures9),13),19). The emulsion can be prepared simple by adding small amounts of aqueous solution of LiCl(0.1 M)into C18AA toluene gel. Viscoelastic measurements of the opaque emulsion phase showed that the complex viscosity increases drastically from 10-1 to 103 Pa・s upon heating. This remarkable viscosity change in fluidity upon heating and cooling is completely reversible.
The amount of LiCl in the gel regulates the rigidity of the heat-induced gel with no influence over the sol-gel transition temperatures. In constrast, the concentration of C18AA determines the transition temperatures - the transition temperatures increase linearly with decreasing concentration of C18AA, as shown in Fig. 3. The existence of this linear relationship over a wide range of temperature(15-55°C)provides opportunities for the potential application of this gel in new useful stimuli-responsive materials.
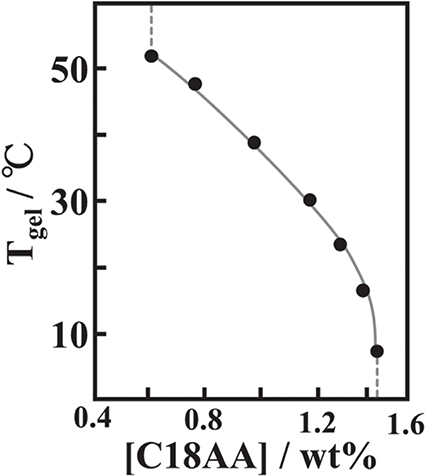
Phase transition temperature of heat-induced gel with C18AA concentration.
A very important question in heat-induced gelation is regarding the origin of the difference in the sol and gel states. In order to investigate the differences in the molecular assembly of C18AA between the sol and gel states, images of the opaque emulsions in the sol and gel states were observed using optical and fluorescence microscopy. Both the opaque phases in the sol and gel states were O/W emulsions and the internal phase fraction remained unchanged over all temperatures. Although the droplets did deform to a non-spherical shape at higher temperatures, the emulsion state remained stable and no phase separation was observed. The high stability of the emulsions at high temperatures indicates the formation of a network structure within the continuous water phase, such as entangled micelles, which is induced by an increase in the concentration of C18AA in the water phase17). Thus, O/W emulsions in the gel state are trapped and pinned by the network of C18AA aggregates formed in the continuous water phase, leading to a high viscosity of the system.
LiCl was found to be essential for the formation of heat-induced gels, but the question whether compounds other than LiCl can be effective in forming heat-induced emulsion gels remains to be answered. We demonstrated that heat-induced gels can also be prepared using HCl, and the sol-gel transition temperatures also decrease linearly with increasing C18AA concentration13). Interestingly, the sol-gel transition of the O/W emulsions was also very sensitive to pH, and it was possible to reversibly control the sol-gel phase transition by changing the pH over many cycles13). The pH range between the sol and gel states was quite narrow. Thus, we have successfully prepared a novel double-stimuli-responsive gel based on O/W emulsions consisting of C18AA and HCl.
2.3 Thermo-responsive coloring in C18AA emulsionsMaterials exhibiting thermo-responsive coloring phenomena have been extensively studied, because they can be used as imaging and sensing materials. During the preparation of C18AA emulsions containing an additive of tetraoctylammonium bromide(TOAB)12), we fortuitously identified a novel thermo-responsive iridescent phenomenon. A water/C18AA+TOAB/toluene system comprising[C18AA]=30 mM, [TOAB]=6 mM and a toluene volume fraction of 0.88 was mixed to form an opaque emulsion at room temperature, whereas upon increasing the temperature above 38°C, the emulsion developed a structural color(Fig. 4). Interestingly, the emulsions maintained its iridescent color even under stirring. Further increasing the temperature above 47°C resulted in the formation of an opaque emulsion and the disappearance of the iridescent color. Significantly, the color development was observed over a narrow temperature range from 38°C to 47°C, and this coloring phenomenon was completely reversible over many cycles of heating and cooling. The reflection spectra of the iridescent emulsion at an incident angle(θ)of 45° showed a sharp peak at 480 nm. The position of this peak shifted to a shorter wavelength with an increase in the incident angle, and the plot of sin2θ against λ2 was linear, which are characteristic features of interference color. This observation implies that the water/C18AA+TOAB/toluene system has a periodical layered structure, and the lattice spacing d of the interference iridescent emulsion is 180 nm.
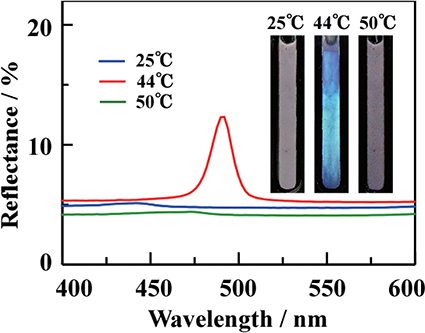
The direct UV-vis reflection spectra of iridescent emulsions in the presence of Au NPs at[C18AA]=30 mM, [TOAB]=6 mM. Inset shows the photographs of the iridescent emulsion.
Electrical conductivity, viscosity and the fluorescence microscopy analysis of the water/C18AA+TOAB/toluene system reveal that the emulsions show a phase inversion from O/W to W/O through the lamellar phase that develops an iridescent color. The iridescent color derived from the lamellar structure is stable for at least a week.
For thermo-responsive coloring materials, the coloring temperature and the color, i.e., the spectral range of the coloring materials, are the essential features. Thus, if we can control independently and on demand the coloring temperature and the color, we can obtain materials with desirable thermo-responsive coloring features. Here, the spectral range of interference color can be tuned by regulating the lattice spacing d of the lamellar structure, whereas the coloring temperature depends on the phase inversion temperatures of the emulsions.
As shown in Fig. 5A, the values of d are proportional to the reciprocal concentration of C18AA, while they are independent of the R value(defined as[C18AA]/[TOAB]). If C18AA molecules are completely consumed by the formation of lamellar layers at the oil-water interface, the number of lamellar layers should increase linearly with increasing concentration of C18AA. The number of the lamellar layers is inversely proportional to the d values for the constant volumes of toluene and water, and thus, the linear relationship between d and[C18AA]-1 is a reasonable result. In contrast, the coloring temperature is shifted to a considerably lower temperature with decreasing R value(Fig. 5B), but is not affected by[C18AA]. This result indicates that the coloring temperature can be regulated by changes in[TOAB]/[C18AA]ratio, i.e., the TOAB concentration for the constant concentration of C18AA. Thus, the coloring temperature and the color can be controlled completely independently by the concentrations of C18AA and TOAB, respectively.
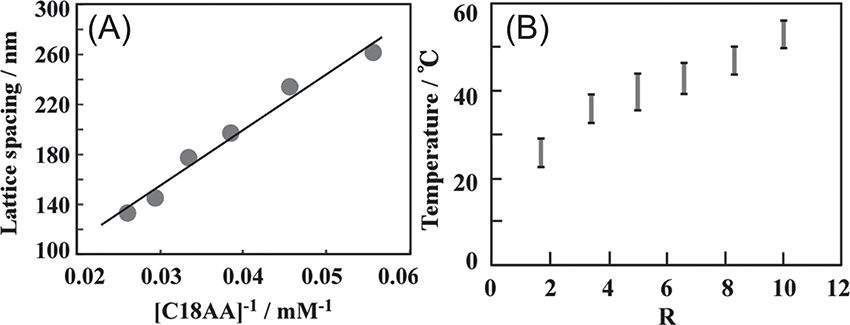
(A)Plot of the lattice spacing d as a function of[C18AA]-1 at[C18AA]/[TOAB]=5 and(B)plot of the coloring temperature range with R at[C18AA]= 30 mM.
One-dimensional noble metal nanowires(NWs)have attracted significant attention due to their unique electrical, optical, and magnetic properties and their potential applications in nanodevices and catalysis23),24). We demonstrate that the lamellar structure of C18AA within toluene gels is extremely suitable as a soft template for the formation of ultrathin and straight Au NWs with diameters of less than 2 nm and lengths of a few micrometers11),16),17),18).
Ultrathin and straight Au NWs(Fig. 6A)are prepared easily by adding an oil-soluble reducing agent into C18AA toluene organogels containing HAuCl4. The lamellar structure of C18AA is vital for the formation of Au NWs, because the reduction of HAuCl4 in C18AA solutions instead of in the gels results in the formation of Au nanoparticles(NPs)only without the formation of Au NWs. Further, high-resolution TEM images revealed that ultrathin Au NWs grow in the(111)direction, because a periodic fringe corresponding to the(111)lattice spacing is observed along the long axis of the Au NWs(Fig. 6B). This result indicates that the side face of Au NWs has a large amount of(100)and/or(110)crystal facets. This result is consistent with the fact that C18AA molecules adsorb on the(100)and(110)crystal facets and hardly adsorb on the(111)crystal facet10),14). Therefore, the selective adsorption property of the terminal amine groups of C18AA onto the(100)or(110)crystal facets of Au is also essential for fabrication of ultrathin Au NWs.
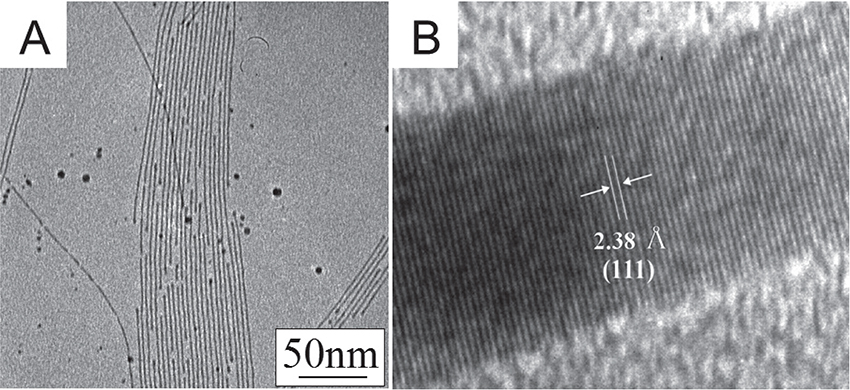
(A)TEM and(B)HR-TEM images of ultrathin Au NWs.
In this section, we show another example of the soft template function of C18AA. Pd NWs and even bimetallic Pd-Ni NWs can be easily prepared using C18AA as a soft template under slow reduction condition22). We also demonstrate that the bimetallic Pd-Ni NWs have a very high catalytic activity for the hydrogenation of 4-nitrophenol to 4-aminophenol compared to Pd NWs, Pd NPs, and Pd-Ni NPs.
Pd NWs are prepared by the following method. A solution of TOAB in toluene is added to an aqueous solution of K2PdCl4 and the mixture is stirred. After the addition of C18AA in the separated toluene phase, an aqueous solution of NaBH4 is added and the system stirred for 24 h at room temperature. Fig. 7A shows the representative TEM images of the resulting network-structured NWs with an average diameter of 3.5±0.4 nm. STEM-EDX and XRD measurements confirmed that the NWs were composed of palladium. Interestingly, the present method produced only Pd NWs without NP byproducts.
In order to confirm the presence of the template, C18AA aggregates in toluene were analyzed before the reduction with NaBH4. As shown in Fig. 7B, a network structure of C18AA aggregates, similar to that of Pd NWs, was observed in the TEM image, indicating that C18AA aggregates formed in toluene act as a soft template for the formation of Pd NWs. The observation that the network structure of C18AA aggregates can be employed in the formation of Pd NWs opens up the possibility of utilizing C18AA to prepare NWs from other metals. We successfully prepared bimetallic Pd-Ni NWs and Pt NWs with average diameters of a few nanometers.
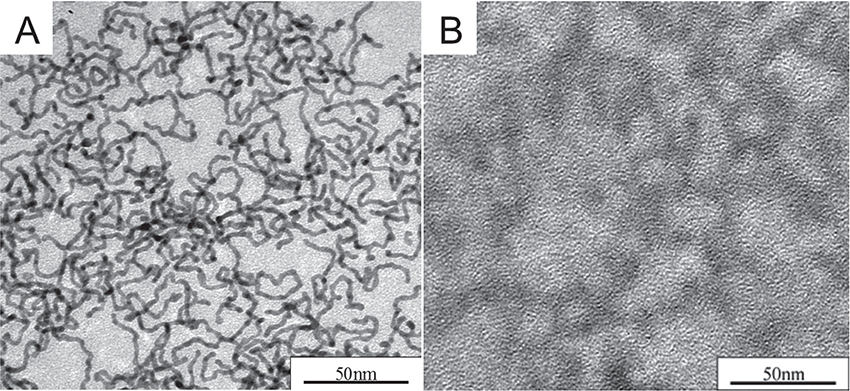
TEM image of(A)Pd NWs and(B)network structure of C18AA aggregates as a soft template.
Pd nanocrystals have been widely used as catalysts for hydrogenation reactions, and their catalytic activity depends strongly on the shape as well as the composition of the nanocrystals. We examined the catalytic activity of Pd NPs, Pd NWs, Pd-Ni NPs, and Pd-Ni NWs using the reduction of 4-nitrophenol to 4-aminophenol by NaBH4 as a model reaction. Pure Ni nanocrystals are known to have very low catalytic activity for the hydrogenation of 4-nitrophenol. The catalytic activity of Pd NWs was found to be higher than that of Pd NPs, even though the surface area per volume of the Pd NWs was smaller than that of the Pd NPs. Interestingly, Pd-Ni NWs has a much higher catalytic activity that Pd NWs, although the moles of Pd atoms in Pd-Ni bimetallic NWs were fewer than in Pd NWs. Comparison of the catalytic activity of Pd-Ni NWs with that of Pd NWs under the same Pd mole condition revealed that the catalytic activity per Pd-mol for Pd-Ni NWs was as much as 7.7 times higher than that of Pd NWs.
We have demonstrated that the multifunctional nature of C18AA with two amide moieties make it suitable for a number of applications that are equivalent to or higher than that of supramolecules with more complex molecular structures. C18AA acts as a high-performance soft material with stimuli-responsive functionalities. C18AA has an organogelator property and forms an aggregate with a lamellar structure in apolar solvents, where the hydrocarbon chains are interdigitated and the adjacent amide groups are interacting through hydrogen bonding. Moreover, we revealed that O/W emulsions of the C18AA/toluene/LiCl system show a heat-induced gelation property, and that the transition temperature can be precisely regulated over a wide temperature range by altering the concentration of C18AA. Another characteristic feature of C18AA emulsions in a water/C18AA+TOAB/toluene system is the development of an iridescent color derived from the interference of the multilayered liquid crystalline structure, where the coloring temperature and the color can be controlled completely independently by the concentrations of C18AA and TOAB, respectively. We have also demonstrated that C18AA has an excellent application as a soft template; ultrathin Au NWs with an average diameter of 1.8 nm and an average length of a few micrometers, and Pd and Pd-Ni NWs were successfully prepared using the self-assembled networked-structure of C18AA aggregates as templates.
This work was supported in part by research grants by JSPS KAKENHI Grant Numbers 25600030, 22651035, 21310063, 19510106, 17510091.