2025 Volume 74 Issue 2 Pages 123-130
2025 Volume 74 Issue 2 Pages 123-130
Biological activities observed in living systems occur as the output of which nanometer-, submicrometer-, and micrometer-sized structures and tissues non-linearly and dynamically behave through chemical reaction networks, including the generation of various molecules and their assembly and disassembly. To understand the essence of the dynamic behavior in living systems, simpler artificial objects that exhibit cell-like non-linear phenomena have been recently constructed. However, most objects exhibiting cell-like dynamics have been found through trial-and-error experiments, and there are no strategies for designing them as molecular systems. This review describes how cell-like dynamics of oil droplets in surfactant solution, such as self-propelled motion, chemotaxis, division, and deformation, are induced by combining molecular properties of system components toward self-propelled microrobots.

Biological activities observed in living systems occur as the output of which nanometer-, submicrometer-, and micrometer-sized structures and tissues non-linearly and dynamically behave through chemical reaction networks, including the generation of various molecules and their assembly and disassembly. Numerous reductive and constructive approaches have found that such a hierarchy in which molecular conversions induce macroscopic dynamics is complicated. Dynein and kinesin are microtubule-based motor proteins that are critical to play an important role in the intracellular transport of substances driven by transforming chemical energy into mechanical energy1),2),3). Cell locomotion is also undoubtedly complex, requiring coordinated activity of cytoskeletal, membrane, and adhesion systems. Amoebic motion is initiated by the front end of the pseudopod being pushed forward by actin polymerization at the front part of the body4).
To understand the essence of the dynamic behavior in living systems, simpler artificial objects that exhibit cell-like non-linear phenomena have been recently constructed in the field of active matter5). This aims to reductively extract and understand the dominant factor in the mechanism of dynamic behaviors observed in living systems by fabrication of self-propelled objects. A typical example is an object with a shape anisotropy, such as Janus particles and vesicles having tiny pores (stomatocytes) . Janus colloids with two different properties on each side of the particle have found widespread applications in the design as active objects. These are self-propelled due to chemical reactions at one side or within themselves6),7),8),9),10). On the other hand, the generation of some anisotropy in observed spaces is required to induce the self-propelled motion of objects without a shape anisotropy. By precisely setting experimental conditions, spherical objects also spontaneously generate the anisotropy surrounding themselves, resulting in a symmetry-breaking to be self-propelled. Because spontaneous symmetry-breaking is necessary to induce the movement of isotropic objects, spherical droplets have drawn considerable attention in nonequilibrium physics. In recent years, there has been much interest in supramolecular chemistry, called systems chemistry11),12) , where attempts are made to create dynamics of droplets by cleverly combining the characteristics of building blocks13),14),15),16). However, most of these dynamics have been found through trial-and-error experiments, and there are no strategies for designing them as molecular systems. If we could create the dynamics observed in living organisms as hierarchical chemical systems based on the molecular properties of building blocks, it would dramatically advance our understanding of living systems and lead to the creation of novel materials with the motility of living systems.
Our research group has focused on spherical oil droplets in surfactant solution and constructed their cell-like dynamics as molecular systems in the field of systems chemistry. This review describes how combining the surfactant and oil molecules induces self-propelled motion, chemotaxis, division, and deformation of oil droplets toward self-propelled microrobots capable of realizing complex mechanical tasks.
The mechanism of the self-propelled motion of oil droplets in a surfactant solution has been interpreted from the mathematical model based on fluid mechanics17),18),19),20). Surfactants can adsorb to the droplet surface from all directions. When relatively large amounts of surfactants locally adsorb onto the droplet surface owing to a stochastic fluctuation, Marangoni flow spontaneously occurs from the area with a lower interfacial tension to that with a higher interfacial tension, forming an inner droplet flow. Then, both the flows at the surface and the interior of the droplet grow to macroscopic convection, and the droplets spontaneously move. If the convection strength is insufficient for self-propelling, the interfacial tension of the droplet surface approaches to equilibrium, and the droplet eventually ceases its motion. However, there were no reports of experimental demonstrations of this mechanism.
We therefore focused on the click reaction, which proceeds with high efficiency without producing any by-products21). We constructed a chemical system in which surfactants with triazole groups (TS) are generated in situ through the Huisgen cycloaddition reaction of hydrophobic alkynes (AL) and hydrophilic azides (AZ) . We also attempted to visualize the convection around the oil droplets before and after they started to move (Fig. 1) . In the presence of Cu (I) , the catalyst for the Huisgen cycloaddition reaction, oil droplets composed of AL and n-heptyloxybenzaldehyde (HBA) in an aqueous solution containing AZ start to move about 10 minutes after the start of observation. When hydrophilic fluorescent microbeads were added to the same sample, the flow field around one droplet was observed before and after it started to move. As a result, we found that tiny flows occurred around the droplets after 6 minutes, and they gradually became stronger. Then, when a sufficiently large convection formed, the oil droplet started to move. From 1H NMR analysis, the amount of TS produced when the oil droplet started to move was estimated to be around 0.2 mM. At this condition, it was revealed according to the pendant drop method that the interfacial tension of aqueous solution containing TS and HBA was significantly lower than that of the aqueous solution without TS. Therefore, we have successfully demonstrated an experimental picture which is proposed by the mathematical model, in which the droplets move due to Marangoni convection based on heterogeneity of interfacial tension on the droplet surface22).

Molecular systems inducing self-propelled motion of oil droplets. The TS generation induces the self-propelled motion of the droplet through the Cu-catalyzed azide-alkyne cycloaddition. Reprinted with permission from reference 22. Copyright 2019 American Chemical Society.
On the basis of the mechanism for self-propelled droplets described above, if the interfacial tension of one side of the droplet is lower than the opposite side, it would be possible to induce behavior similar to the taxis of living organisms. Based on this strategy, we have investigated inducing the taxis behaviors of oil droplets in the gradient of pH and the concentration of specific chemical species.
We have reported some taxis behaviors of droplets in the linear-type microchannel. First, the behavior of HBA oil droplets was observed an emulsion containing a gemini-type cationic surfactant having a carbonate linkage in a microfluidic device (the top in Fig. 2A) . We found that, when the NaOH solution of high concentration was added from one side of the device into the emulsion at a constant rate, immobile oil droplets started to move in the direction of a higher concentration of NaOH. This was thought to be because the hydrolysis of carbonate linkages in the high pH region generated components with different surfactant properties, preferentially adsorbed to one side of the oil droplet, inducing the directional motion, that is, the positive taxis of droplets. However, since the hydrolysis of carbonate linkages was so rapid under the basic conditions, the directional behavior lasted only a few seconds23). Therefore, instead of a hydrolysis reaction of components, we focused on a neutralization reaction that could adjust the amount of product according to the pH (the bottom in Fig. 2A) . This study investigated the pH-sensitive motion of oil droplets in a surfactant solution containing fumaric acid derivatives, which act as regulators in both acidic and basic conditions, in a linear-type channel to create pH gradients. Specifically, in the pH gradients ranging from acidic to basic conditions, HBA droplets exhibited positive chemotaxis, moving toward higher or lower pH regions, for several minutes. The carboxylic acid and amine groups of fumaric acid derivatives were neutralized under varying pH, generating surface-active compounds that influence the interfacial tension of the oil droplets. This heterogeneity in interfacial tension was a driving force for the droplets to move toward areas with higher pH or lower pH24). When a regulator having an aniline moiety was utilized, the oil droplets also showed positive chemotaxis towards concentration gradients of metal ions, such as nickel (II) and copper (II) (Fig. 2B) . After stopping, they underwent a structural transition to vesicles. The series of dynamics was presumed due to the complexation of the metal ion with the aniline moiety, the difference in the interfacial tension of the complex from that of the original surfactant, and the vesicle-forming ability of the complex25). In this way, we have created a chemical system in which the chemotaxis of oil droplets is induced by using an amphiphilic molecule with a reactive site as a regulator.

Molecular systems inducing chemotaxis of oil droplets in a linear-type channel. (A)Chemotaxis of droplets in the pH gradient using hydrolyzable gemini-type cationic surfactants(top)and fumaric acid derivatives(bottom). Reprinted with permission from reference 23. Copyright 2019 American Chemical Society. (B)Chemotaxis and structural transition of droplets in the presence of cationic surfactants having an aniline moiety. Reprinted with permission from reference 24. Copyright 2022 John Wiley and Sons.
It was also succeeded in inducing phototaxis, in which HBA droplets in a cationic surfactant solution were self-propelled to escape from the direction of light illumination, a minimally invasive stimulus that does not significantly change the observation system. To induce the phototaxis of self-propelled oil droplets, we focused on gemini-type cationic surfactants having an azobenzene moiety. The HBA droplets exhibit negative phototaxis, indicating that they move away from the light source upon ultraviolet (UV) illumination. When exposed to UV light, the photoisomerization from a trans-isomer to a cis-isomer of the azobenzene gemini-type surfactants immediately occurred at the side of UV illumination because HBA completely adsorbed UV light. This resulted in the local interfacial tension becoming high, affecting the droplet motion. Therefore, the droplets moved rapidly in the opposite direction of the UV light, changing their motion directions within seconds. Even low-intensity UV light caused the stationary droplets to start moving, with higher intensities accelerating the response. This sensitive phototaxis was achieved by combining molecular properties of a gemini-type cationic surfactant with an azobenzene skeleton and HBA26).
In addition, other unique dynamics of HBA droplets was observed when a light-responsive surfactant was changed from a gemini-type to a single-chain type AzoTAB (Fig. 3A) . Under UV illumination from the vertically upward direction, a phenomenon was observed in which droplets form a circular pattern while clustering together (Fig. 3B) . From an analysis of the flow field around the droplets and an evaluation of the physico-chemical properties of the surfactant, the mechanism of this phenomenon was estimated as follows. Because HBA absorbs UV light, the photoisomerization of AzoTAB mainly progressed on the UV-illuminated side of the droplet surface. Since an interfacial tension gradient was formed, a flow from the bottom to the top of the droplet formed. When the two oil droplets were relatively close together, the flow between the two droplets canceled each other out and weakened, while the flow field on the outside became stronger. This caused the droplets to move closer together. In addition, the critical micelle concentration of AzoTAB increased due to the isomerization of azobenzene from a trans-isomer to a cis-isomer. Thus, the number of micelles decreased, and the amount of solubilized oil also decreased. Therefore, the oil components were present in the aqueous phase, and it was thought that they formed a pattern by being deposited in a circular shape due to the flow field generated around the droplets (Fig. 3C) . In this way, the collective motion and pattern formation can be induced by a hierarchical chemical system based on the photoisomerization of azobenzene, which changes the properties of the surfactant and creates a characteristic flow field27).
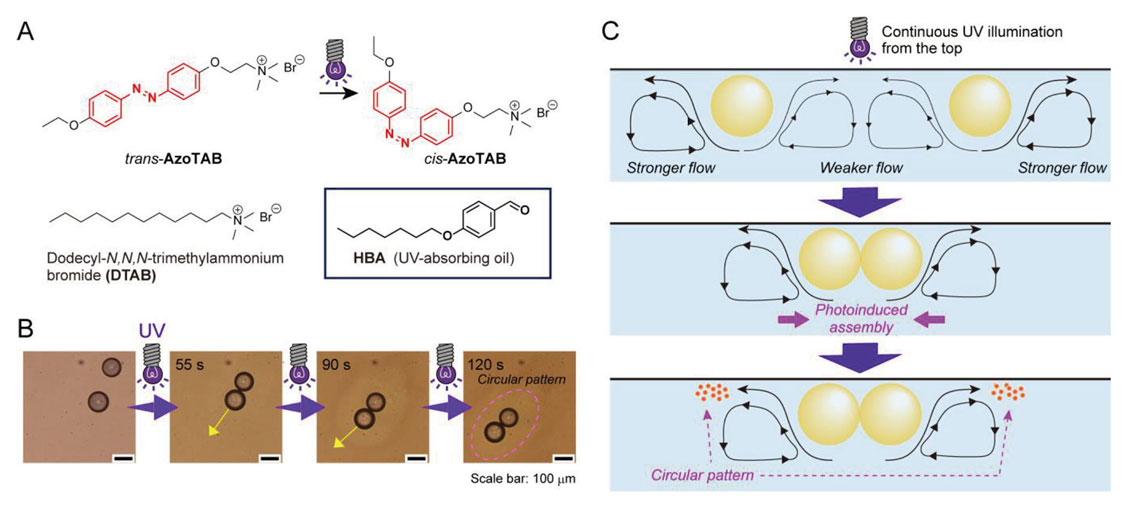
Molecular system inducing photoinduced assembly of droplets and concurrent pattern formation. (A)Molecular structures of surfactant and oil compounds. (B)Sequential micrograph of droplets under UV illumination. (C)Schematic illustration of the proposed mechanism of the droplet dynamics. Reprinted with permission from reference 27. Copyright 2023 Elsevier.
These taxis behaviors propose that the rapid response of these oil droplets can be helpful for applications that require precise control over the movement of micrometer-sized objects, such as in targeted delivery systems. Additionally, these systems mimic living organisms to adapt to external stimuli, offering insights into how such systems might be used to investigate the behavior of artificial cells or micro-scale robots.
Living organisms exhibit not only taxis but also other unique non-linear phenomena, such as division, deformation, and transformation. We have also induced such characteristic dynamics of oil droplets based on the molecular properties of building blocks.
The dynamics of self-propelled oil droplets coupling with their division was found using a mixture of HBA and decanol as oil droplet (Fig. 4A) . The division of droplets occurred when the chemical components within the system underwent a dehydrocondensation reaction in the oil droplet, leading to acetal formation. The droplet motion and division were influenced by surfactant concentration, droplet composition, and experimental conditions such as pH and ionic strength. At the specific concentration conditions of cationic surfactant and HCl, the droplets exhibited repeated divisions, producing smaller daughter droplets. These daughter droplets also moved autonomously and eventually dissolved in the solution. We proposed a four-stage mechanism for the division and movement of the droplets. As the droplets move, they took up additional surfactants and water, leading to the formation of smaller daughter droplets inside the larger mother droplet. Once the mother droplet became packed with the daughter droplets, it divided, and the process repeated28). In addition, the division of self-propelled oil droplets composed of HBA and decanol was induced by the hydrolysis of the five-membered acetal-containing cationic surfactant, which supplied oil components into the droplet (Fig. 4B) . The division started as a surfactant having a five-membered acetal hydrolyzed in the acidic solution. This hydrolysis produced fresh HBA, which was taken into the moving droplets. Tiny daughter droplets generated and grew inside the droplet as they took in more HBA. As the daughter droplets increased in size, the mother droplet became packed and eventually burst, releasing the daughter droplets. These newly generated droplets continued to move independently. The division process depended on the balance of surfactant and oil amounts, as well as the concentration of HCl. Optimal conditions allowed for the continuous division and self-propelled motion of the daughter droplets, demonstrating a primitive model of self-reproducing chemical systems29). These studies contribute to understanding the dynamics of inanimate molecular systems replicating cell-like behaviors. Therefore, they provide a deeper understanding of the mechanisms behind self-propelled droplets and their potential applications, such as the development of artificial life systems, offering insights into non-equilibrium chemical systems.
By using a mixture of undecanal and decanol, it was found that oil droplets exhibited self-propelled motion while deforming, mimicking amoeboid motion underwater (Fig. 4C) . Their movement and deformation were influenced by surfactant concentration and the presence of electrolytes. The deformation of oil droplets was associated with the interaction between the surfactant and oil molecules, primarily through interfacial tension imbalances. In other words, the deformation mechanism was influenced by the localized accumulation of surfactant molecules at different points on the droplet surface. This caused the droplets to exhibit shape changes, often becoming elliptical. The direction of deformation was typically perpendicular to the direction of movement, with a time delay of about 0.1 seconds between the velocity of the droplet and the onset of deformation. This delay suggested that the deformation was linked to both diffusive transport of surfactants and convective flow around the droplet, creating a dynamic interplay that induced both the motion and shape changes of the droplets30).
Furthermore, it has succeeded in inducing the dynamics of self-propelled oil droplets and their transformation into vesicles in an acidic surfactant solution (Fig. 4D) . The system used oil droplets composed of an imine-containing oil component and a cationic surfactant at low pH. The imine-containing oil underwent hydrolysis in the acidic solution, producing two components: decylamine, which acted as a surfactant at a low pH, and HBA, which became the active oil component. Initially, flocculated particles formed, which gradually transformed into spherical oil droplets caused by the production of decylamine through hydrolysis. These oil droplets exhibited self-propelled motion due to the difference in the interfacial tension. The movement continued for around 30 minutes, eventually transforming membranous figures. Over time, these membranous figures evolved into micrometer-sized vesicles31). The system provided insights into the linkage between self-propelled oil droplets and protocell-like vesicle formation, demonstrating how molecular dynamics in a non-equilibrium system can lead to complex transformations. The finding was also relevant to understanding primitive cell models and could have applications in creating nonenzymatic metabolic systems.

Molecular systems inducing the unique cell-like dynamics of oil droplets. (A)Sequential micrographs of the division of self-propelled oil droplets composed of HBA and decanol. White arrows indicate the self-propelled motion. Scale bar: 20 µm. Reprinted with permission from reference 28. Copyright 2015 Royal Society of Chemistry. (B)Sequential micrographs of the division of self-propelled oil droplets in the solution of five-membered acetal-containing surfactants. Arrows indicate the self-propelled motion of each droplet. Scale bar: 20 µm. Reprinted with permission from reference 29. Copyright 2015 American Chemical Society. (C)Sequential micrographs of the division of self-propelled oil droplets composed of undecanal and decanol. White arrows indicate the self-propelled motion. Scale bar: 30 µm. Reprinted with permission from reference 30. Copyright 2016 Springer Nature. (D)Schematic illustration of the transformation of oil droplets composed of imine-containing oil components. Reprinted with permission from reference 31. Copyright 2016 American Chemical Society.
We have recently found that the motion mode of oil droplets varies significantly depending on the combination of surfactant and oil molecules32). Analyzing dynamics of oil, water, and surfactant molecules at the droplet surface by molecular simulations, it was suggested that the adsorption rate of surfactants to the droplet and intermolecular interactions between surfactant and oil molecules are associated with the motion speed of oil droplets33). These attempts are expected to lead to the development of a new molecular chemistry that enables the creation of macroscopic droplet motion as a molecular system based on the molecular properties of building blocks.
This review has introduced our previous work on the induction of the nonlinear phenomena of droplets in surfactant solutions as a molecular system with a hierarchical structure in which molecular transformation, changes in the properties of building blocks, and changes in the microstructure are interlocked. The dynamics of adsorption and desorption of surfactants at the oil-water interface can lead to control of the oil-water interface and contain essential findings that can also be used to elucidate natural phenomena such as convection around the interface. The current molecular approach includes the concept of nanoarchitectonics, which constructs functional material systems by architecting atoms, molecules, and nanostructures as building blocks34). Our nanoarchitectonics is thus expected to pave the way for various applications, such as biomimetic technology that converts chemical energy into mechanical energy, micro-transport carriers that incorporate useful hydrophilic substances into droplets, micro-reactors, and shape-changeable micro-probes. These new functional materials will improve the constantly changing chemical environment, such as the body and natural environment, due to their advanced responsiveness that can change their properties accordingly and demonstrate the functions most suited to that environment.
This study was partially supported by JSPS KAKENHI (Grant No. JP20H02712) and JSPS Japan–Hungary Bilateral Joint Research Project (JPJSBP120213801) .
The authors declare there are no conflicts of interest.