2023 Volume 65 Issue 4 Pages 270-274
2023 Volume 65 Issue 4 Pages 270-274
Purpose: This study investigated the effectiveness of curcumin-based antimicrobial photodynamic therapy (aPDT) against Staphylococcus aureus (S. aureus), the causative agent of ventilator-associated pneumonia.
Methods: Curcumin was added to S. aureus culture medium at concentrations of 25, 2.5, and 0.25 µM. After 60 min (20-25°C), each culture was irradiated for 1 and 3 min, and viable bacteria were counted. Curcumin (25 µM) was also added to a bacterial suspension with D-mannitol and sodium azide; microbial counts were determined after irradiation for 3 min.
Results: S. aureus was significantly reduced in the 1-min (P = 0.043) and 3-min (P = 0.011) irradiation groups in comparison to the 0-min irradiation group with 25 µM curcumin. No significant differences were observed between the curcumin alone group and the curcumin plus D-mannitol or sodium azide group.
Conclusion: The findings of this study indicate that prolonged exposure (≥1 min) of S. aureus to LED in 25 μM curcumin solution induces cell wall injury. Curcumin-based aPDT as an adjunct to conventional oral care, employing existing dentistry equipment, offers a promising approach that does not rely on antimicrobial drugs or allows the emergence of resistant bacterial strains. This suggests its potential role in future strategies aimed at preventing ventilator-associated pneumonia.
Among the healthcare-associated infections of global concern, ventilator-associated pneumonia (VAP) is one of the most common infectious complications in intensive care units, with a mortality rate of 50-70% [1]. VAP is a type of nosocomial pneumonia that develops more than 48 h after the start of ventilation under intubation and is frequently associated with Staphylococcus aureus (S. aureus) [2], reported to be the most common causative organism [3]. The upper airway consists of the nasal cavity, pharynx, and larynx, and is responsible for heating and humidifying inspiratory air and removing foreign bodies. Therefore, patients who are on ventilators have a 6-20-fold higher risk of developing pneumonia than those who are not intubated, since microorganisms can enter the lower respiratory tract by bypassing the upper respiratory tract owing to the placement of an artificial airway, such as a tracheal or a tracheostomy tube [4]. For the treatment of VAP, antimicrobial agents should be administered after assessing the disease severity and risk of bacterial resistance [5]. Although seven-day antimicrobial treatment is recommended for most patients with VAP [5], in practice, patients are often treated with broad-spectrum antimicrobials for a prolonged period or a combination of different antimicrobials, which may create problems involving multidrug-resistant bacteria and other adverse effects. Approximately 30% of patients on ventilators develop VAP, mainly due to carbapenem-resistant bacteria [6].
Appropriate preventive measures for VAP are essential, and these include proper head elevation [7], suctioning the upper cuff of the tracheal tube, selective oral and digestive decontamination [7], oral care with chlorhexidine [8], and mechanical plaque removal (e.g. tooth brushing) [9]. However, no reliable preventive measures have yet been established. For example, oral care using chlorhexidine is crucial as a VAP preventive measure [10]; however, it may result in mucosal ulcers when used at high concentrations or problems involving microbial resistance [11].
Photodynamic therapy (PDT) has long been used to selectively kill tumor tissue in cancer treatment [12]. Antimicrobial photodynamic therapy (aPDT) is a treatment that uses photosensitive substances (PS), which cause excitation and injury to pathogenic bacteria [13]. Therefore, applying aPDT can hinder the development of resistant bacteria, thereby effectively preventing VAP.
Curcumin, a light-sensitive substance, is the main component of turmeric and is classified as a curcuminoid, a type of polyphenol [14]. It has a wide range of biological activities, including antiviral, anti-inflammatory, anti-tumor, and antibacterial activities [15], and has attracted attention as a PS for aPDT [16]. Therefore, the aim of the present study was to investigate the effectiveness of curcumin-based aPDT in antimicrobial-free oral care for prevention of VAP.
S. aureus ATCC 12600 strains, stored in the Department of Oral Microbiology, Kanagawa Dental University, were used. Cultures were first incubated in Brain Heart Infusion (BHI) broth (Becton Dickinson Co., Franklin Lakes, NJ, USA) with yeast extract (5 mg/mL, Becton Dickinson Co.), hemin (5 µg/mL, Fujifilm Wako Pure Chemical Corp., Osaka, Japan), and vitamin Kl (10 µg/mL, Fujifilm Wako Pure Chemical Corp.). The cultures were then inoculated onto BHI-YHK medium for 18 h at 37°C under aerobic conditions.
Adjustment of reagentsCurcumin (Fujiflm Wako Pure Chemical Corp.) was dissolved in dimethyl sulfoxide (DMSO, Fujiflm Wako Pure Chemical Corp.) to obtain concentrations of 250, 25, and 2.5 µM.
Measurement of curcumin absorption wavelengthsCurcumin at 250 µM was diluted in phosphate-buffered saline (PBS, pH 7.4, Shimadzu Diagnostics Corp., Tokyo, Japan) to a concentration of 2.5 µM. The peak absorption wavelength was measured using a DS-11 spectrophotometer (Scrum Inc., Tokyo, Japan).
Bacterial preparationS. aureus cultures were collected by centrifugation at 10,000 rpm for 3 min, after which the bacteria were washed with PBS and adjusted to an absorbance of 1.0 at a wavelength of 550 nm.
Antimicrobial efficacy against bacterial suspensionsTo 200 µL of the prepared bacterial suspension, 2 µL of curcumin was added to obtain final concentrations of 25, 2.5, and 0.25 µM. After 60 min at room temperature (20-25°C), the suspensions were irradiated for 1 and 3 min. Light irradiation was performed using an LED light source device (KTL-100, Kenko Tokina Corp., Tokyo, Japan) with a color filter (B-410, Kenko Tokina Corp.). Bacterial suspensions were diluted and smeared on BHI-YHK agar plates incubated under aerobic conditions at 37°C. The number of viable bacteria (CFU/mL) was determined based on the number of colonies on the plate.
Electron microscopyThe bacterial structure was confirmed using scanning electron microscopy. Curcumin solution was added to the prepared bacterial suspension to a final concentration of 25 µM. After 60 min at room temperature (20-25°C), the suspension was irradiated for 3 min. The bacteria were again collected by centrifugation at 10,000 rpm for 3 min and fixed with 1% glutaraldehyde (pH 7.2, Fujiflm Wako Pure Chemical Corp.) dissolved in 0.1 M cacodylic acid (Fujiflm Wako Pure Chemical Corp.) for 1 h. After fixation, the cells were washed three times with 0.1 M cacodylic acid and dehydrated stepwise (50%, 70%, 80%, 90%, and 100%, 15 min each) using ethanol (Fujiflm Wako Pure Chemical Corp.). Platinum ion sputter coating was then performed using an auto fine coater JFC-1300 (JEOL Ltd., Tokyo, Japan) and observed using a JCM 6000 Plus NeoScope scanning electron microscope (JEOL Ltd.) at an acceleration voltage of 15 kV.
Germicidal effect of 410-nm LED light and curcumin in combination with mannitol and sodium azideCurcumin was added to 170 µL of the prepared bacterial suspension to a final concentration of 25 µM. D-mannitol (Fujiflm Wako Pure Chemical Corp.) and sodium azide (Fujiflm Wako Pure Chemical Corp.) were added to a final concentration of 100 mM. After 60 min at room temperature (20-25°C), the suspension was irradiated for 3 min. Light irradiation was performed using an LED light source device (KTL-100, Kenko Tokina Corp.) with a color filter (B-410, Kenko Tokina Corp.). Bacterial suspensions were diluted and smeared on BHI-YHK agar plates. The bacteria were incubated under aerobic conditions at 37°C, and the number of viable bacteria (CFU/mL) was determined based on the number of colonies.
Statistical analysisAll experiments were performed three times in triplicate (n = 9). The normality of the data was assessed using Shapiro-Wilk test. In instances where the assumption of normality was violated, Kruskal-Wallis test, a non-parametric test, was employed. Subsequent post-hoc pairwise comparisons were conducted using Mann-Whitney test with Bonferroni correction. IBM SPSS Statistics for Windows version 28.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. The significance level was set at P < 0.05.
The absorption peak wavelength in the visible light region was approximately 370 nm. Accordingly, an LED light source with a 410-nm color filter was considered appropriate as a light source including the peak absorption wavelength.
Antimicrobial effect of 410-nm LED light in combination with curcuminInitially, normality was assessed using the Shapiro-Wilk test, revealing a lack of normal distribution (P < 0.001). Therefore, the Kruskal-Wallis test was conducted, demonstrating a statistically significant difference (P < 0.001). The results of the subsequent multiple comparisons are shown in Table 1. The survival of S. aureus was significantly reduced in the 1-min (P = 0.043) and 3-min (P = 0.011) irradiation groups in comparison to the 0-min irradiation group with 25 µM curcumin. In the groups without curcumin and with 0.25 µM, 2.5 µM and 25 µM curcumin, there was a tendency for the number of bacteria to decrease with increasing irradiation time in all groups relative to the median, although the differences were not significant. Comparison of curcumin concentrations showed no significant differences in the survival of S. aureus between the non-irradiated groups with different curcumin concentrations. In the curcumin-exposed groups overall, there was a tendency for a decrease in the number of bacteria as the curcumin concentration increased relative to the median, but none of the differences were significant.
| Irradiation time (min) |
Curcumin concentration (μM) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.25 | 2.5 | 25 | |||||
| P value | P value | P value | P value | |||||
| 0 | 7.80 (7.55-8.05) | 6.20 (5.80-6.25) | vs. 0 μM/0 min P = 1 |
7.40 (6.50-7.40) | vs. 0 μM/ 0 min P = 1 |
5.60 (5.45-6.05) | vs. 0 μM/0 min P = 1 |
|
| 1 | 5.60 (5.55-6.20) | vs. 0 μM/0 min P = 1 |
5.20 (5.15-6.00) | vs. 0 μM/0 min P = 1 vs. 0 μM/1 min P = 1 vs. 0.25 μM/0 min P = 1 |
0.88 (0.87-0.90) | vs. 0 μM/0 min P = 0.439 vs. 0 μM/1 min P = 1 vs. 2.5 μM/ 0 min P = 1 |
0.0022 (0.0021-0.0028) | vs. 0 μM/0 min P = 0.043* vs. 0 μM/1 min P = 0.913 vs. 25 μM/0 min P = 1 |
| 3 | 4.80 (4.65-4.95) | vs. 0 μM/0 min P = 1 vs. 0 μM/1 min P = 1 |
4.60 (3.55-4.65) | vs. 0 μM/0 min P = 1 vs. 0 μM/3 min P = 1 vs. 0.25 μM/0 min P = 1 |
0.13 (0.11-0.14) | vs. 0 μM/0 min P = 0.145 vs. 0 μM/3 min P = 1 vs. 2.5 μM/0 min P = 0.553 |
0.00038 (0.00035-0.00043) | vs. 0 μM/0 min P = 0.011* vs. 0 μM/3 min P = 1 vs. 25 μM/0 min P = 0.493 |
Data are shown as the median (interquartile range) of 1 × 108 CFU/mL. Asterisks indicate P values for differences from control (0 μM/0 min): *P < 0.05
Electron microscopy images of the control (0 μM/0 min) indicated a cell diameter of approximately 0.5-1.5 µm for S. aureus (Fig. 1A). Electron micrographs of S. aureus irradiated with LEDs without curcumin (0 μM/3 min) (Fig. 1C) or treated with 25 µM curcumin but without LEDs (25 μM/0 min) (Fig. 1B) demonstrated no morphological changes. However, cell wall damage was evident in S. aureus treated with 25 µM curcumin and irradiated with LED (25 μM/3 min) (Fig. 1D).
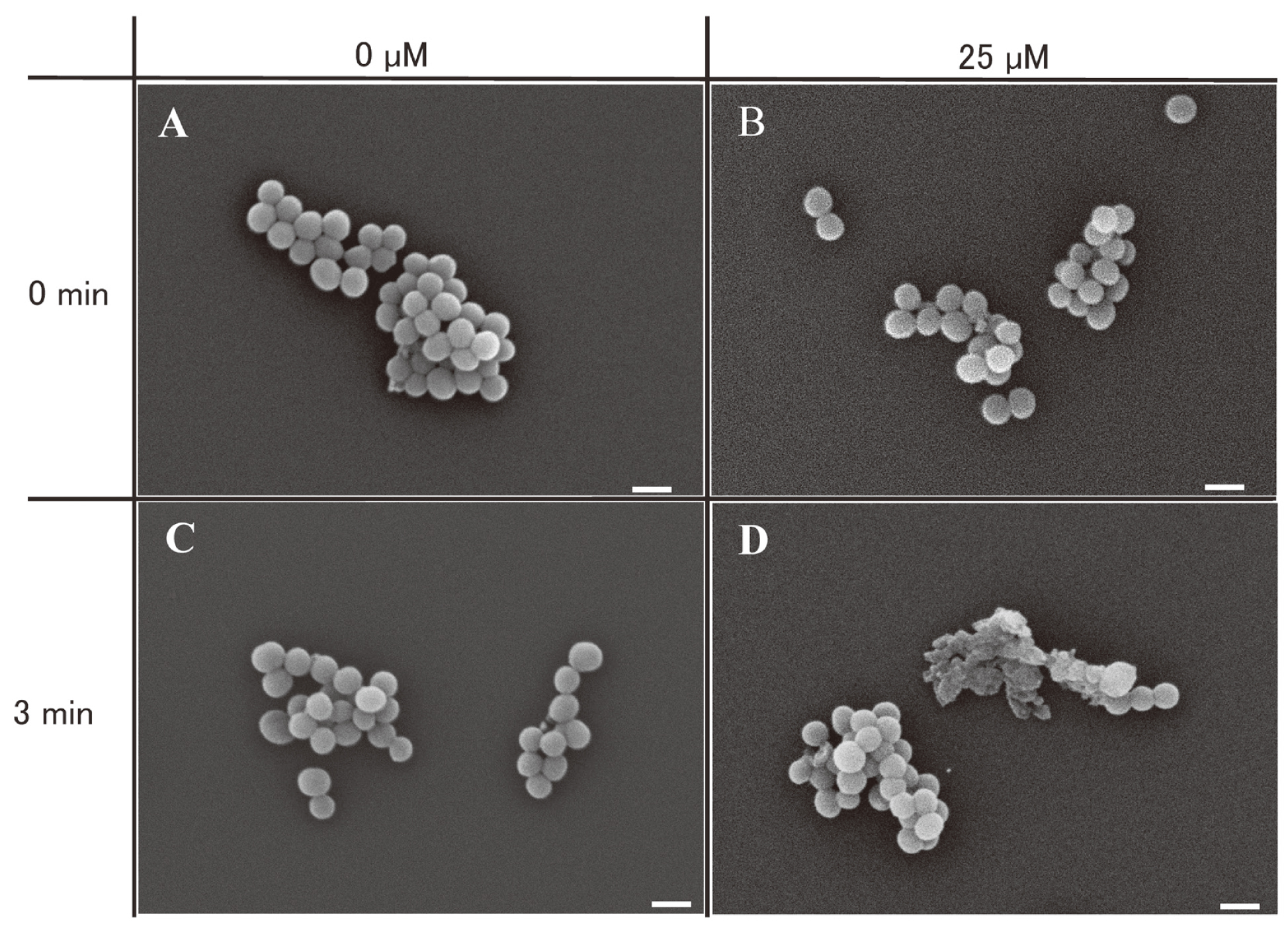
Electron microscopy images depicting the morphology of S. aureus cells subjected to curcumin treatment and LED irradiation. A: without curcumin or LED treatment; B: with curcumin treatment, without LED, C: without curcumin, with LED treatment, D: with curcumin and LED treatment, scale bars: 1 µm
The data were first evaluated for normality using the Shapiro-Wilk test, which revealed a lack of normal distribution (P < 0.001). Consequently, the Kruskal-Wallis test demonstrated a statistically significant difference (P = 0.019). The subsequent multiple comparisons are presented in Table 2. In the non-LED-irradiated groups, no significant differences in bacterial counts were observed among the curcumin-only, curcumin plus mannitol, and curcumin plus sodium azide groups. Comparison of the LED-irradiated groups revealed a trend for increased bacterial counts in the curcumin plus mannitol group relative to the curcumin-only and curcumin plus sodium azide groups based on the median; however, these differences did not reach statistical significance.
| Solution | LED irradiation | |||
|---|---|---|---|---|
| LED (−) | LED (+) | |||
| P value | P value | |||
| Curcumin | 4.60 (4.40-5.45) | 0.0038 (0.0021-0.0055) | vs. curcumin LED (−) P = 0.292 |
|
| Curcumin + mannitol | 4.50 (4.35-4.70) | vs. curcumin LED (−) P = 1 |
0.0041 (0.0038-0.0052) | vs. curcumin LED (+) P = 1 vs. curcumin + mannitol LED (−) P = 0.937 |
| Curcumin + sodium azide | 4.00 (4.00-4.20) | vs. curcumin LED (−) P = 1 |
0.00063 (0.00039-0.019) | vs. curcumin LED (+) P = 1 vs. curcumin + sodium azide LED (−) P = 1 |
Data are shown as the median (interquartile range) of 1 × 108 CFU/mL.
The mechanism of aPDT is characterized by the fact that it does not produce resistant bacteria [13] – type I and II photochemical reactions occur when a PS is irradiated with excitation light, resulting in reactive oxygen species (ROS) and bacterial injury [17]. Photochemical reaction type I involves electron transfer, producing superoxide and hydroxyl radicals. Photochemical reaction type II involves energy transfer and produces excited singlet oxygen. Hydroxyl radicals and singlet oxygen are ROS, which can damage almost all biomolecules (proteins, lipids, and nucleic acids), and therefore kill cells [18]. The optimal excitation light is dependent on the PS; however, in this study, blue light with a maximum absorption wavelength of 410 nm in the visible light range was used because the peak wavelength of curcumin is 370 nm, which is within the UV light range. Curcumin was found to exert bactericidal activity against S. aureus when irradiated with 410-nm LED light. Furthermore, concentrations of curcumin above 25 µM and LED irradiation durations of more than 1 min significantly reduced the number of viable bacteria. Curcumin was first shown to have antibacterial activity against S. aureus, Salmonella paratyphi, Trichophyton gypseum, and Mycobacterium tuberculosis in 1949 [19], and has also been studied for treatment of various other diseases in view of its anti-inflammatory, antioxidant, and apoptosis-promoting properties [20]. Other antimicrobial effects of curcumin, such as anti-candida [21] and antimalarial effects [22], have also been reported.
In the present study, treatment with curcumin alone did not reduce the bacterial count significantly in comparison with the absence of curcumin. However, when LED irradiation was applied in the presence of curcumin, a significant decrease in the viable cell count was observed at a curcumin concentration of 25 µM with a LED irradiation duration of at least 1 min. LED irradiation for more than 5 min is required for antimicrobial action [23]. The significant reduction in the viable bacterial count at 1 min of irradiation observed in the present study may have potential clinical applications. Furthermore, previous studies have reported the bactericidal effect of 405-nm visible light on S. aureus even without PS [24]. In the present study, the curcumin-free group showed a tendency for a reduction in the viable bacterial count after 3 min of LED irradiation, in comparison to the non-irradiated group.
Curcumin-induced aPDT has been reported to have antimicrobial activity against Streptococcus mutans [25], Escherichia coli [26], and S. aureus [23, 27]. However, its mode of action is not clear. Generally, the main bactericidal activity of aPDT is considered to involve type II singlet oxygen [28] derived from photochemical reactions among ROS. However, previous studies of the bactericidal activity of aPDT with curcumin against S. aureus have focused on singlet oxygen [29], hydroxyl radicals [30], and hydrogen peroxide [31], and it is unclear which of these ROS contributes most to the bactericidal activity. Furthermore, one report [32] has suggested that hydroxyl radicals, rather than singlet oxygen, are responsible for the bactericidal effect of blue light irradiation on polyphenols. Curcumin is also a type of polyphenol. Therefore, in the present study, mannitol and sodium azide, free radical scavengers of type I and II reactions, were also used to investigate the main factor of bactericidal activity. The results indicated that inclusion of mannitol tended to impede the bactericidal efficacy of curcumin under LED irradiation, implying the involvement of type I reactions in curcumin-induced aPDT. However, although no statistically significant differences were observed between curcumin alone and curcumin plus mannitol, a tendency for a reduction in the number of viable bacteria was observed. Therefore, to derive more robust conclusions, future investigations should consider expanding the sample size. It is noteworthy that while significant differences were detected in the Kruskal-Wallis test, no significant differences were observed in multiple comparisons. This is a plausible outcome since multiple comparisons involve pairwise comparisons, which are calculated differently from the Kruskal-Wallis test.
There are three possible mechanisms of microbial damage caused by ROS generated by aPDT: damage to cell membranes, inhibition of enzyme activity, and DNA damage [33]. ROS oxidize microbial biomolecules and cause biological damage [34]. Danning et al. reported that photodynamic inactivation of S. aureus by curcumin involved direct DNA and protein damage [35]. However, Rauf et al. [36] considered that the first target of ROS in organisms is the plasma membrane, specifically the lipid bilayer. A previous study [16] has demonstrated cell wall injury by curcumin and curcumin-induced aPDT, and such cell wall injury by curcumin-induced aPDT was also confirmed in the present study. It is suggested that the ROS produced by LED irradiation of curcumin may damage the cell wall, and that this may be responsible for the bactericidal effect. Although sodium azide is used as a preservative [37], the present study employed a concentration that would not have reduced the number of viable cells without LED irradiation. Addition of mannitol did not reduce the number of viable cells. In contrast, however, the inclusion of sodium azide tended to attenuate the population of viable cells under LED irradiation. This observation suggests that the bactericidal effect of curcumin for aPDT may be based primarily on the generation of hydroxyl radicals through type I reactions, rather than type II reactions. However, further investigation is warranted to clarify this mechanism conclusively.
The present study did not examine the effect of aPDT on biofilms. Control of biofilms formed in the oral cavity and tracheal tube is essential for prevention of VAP, and the effects of aPDT on such biofilms will need to be investigated in future studies. The effect of aPDT using methylene blue and red light on biofilm formation in tracheal tubes has been reported [38]. However, although methylene blue has been used in the treatment of methemoglobinemia and cyanide poisoning, it has been shown to have neurotoxic activity and the mechanism of responsible for this remains unknown [39]. In contrast, curcumin, which was used as a photosensitive substance in the present study, is a natural plant-derived compound also used in food, making it very safe for use in the oral cavity.
This study investigated the antimicrobial efficacy of curcumin-based aPDT on S. aureus, which is the organism most commonly implicated in VAP. However, further studies of effectiveness against other causative organisms of VAP will also be needed. In addition, blue LEDs were used in this study. One possible clinical application would be to administer curcumin in some form, such as a paste, in the oral cavity and irradiate it with LEDs. Regarding the effects of blue light on normal cells, a previous study [40] showed that blue light irradiation produces reactive oxygen species in vascular smooth muscle cells, induces oxidative stress-related cytotoxicity, and induces lipid peroxidation and apoptosis. In addition, it was shown that administration of N-acetyl-L-cysteine, a typical intracellular antioxidant, prevents oxidative stress-induced cytotoxicity due to blue light irradiation. Therefore, the side effects of LED irradiation on normal tissues may be prevented by administration of N-acetyl-L-cysteine, for example. Light irradiators used in hospital dentistry for composite resin curing during dental treatment would be applicable for aPDT.
In the present study, curcumin-based aPDT showed significant antimicrobial activity against S. aureus. This suggests that LED irradiation of 25 µM curcumin solution for 1 min would induce cell wall damage, thus contributing to its bactericidal effect. Since oral care to prevent VAP requires mechanical plaque removal such as tooth brushing, curcumin-based aPDT has potential for adjunctive prophylaxis. For actual clinical application of curcumin in the oral cavity, further consideration must be given to the form of curcumin and optimal irradiation conditions. However, curcumin-based aPDT could be added to conventional oral care using equipment currently used in dentistry. This could contribute to VAP prevention without relying on antimicrobials or running the risk of generating resistant bacteria.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this manuscript.