2024 年 66 巻 1 号 p. 9-14
2024 年 66 巻 1 号 p. 9-14
Purpose: The aim of this study was to measure residual monomer, cell adhesion, and cell viability of 3-dimensional printable permanent resin (PR), hybrid ceramic block (HCB), and indirect composite (IC) produced with additive, subtractive, and conventional techniques.
Methods: Five 8 × 8 × 2 mm3 samples of each material were prepared for each experiment. In a 24-h period, monomer release was analyzed with high-performance liquid chromatography, and cell viability and adhesion were evaluated with the water-soluble tetrazolium salt test. Data were analyzed with IBM SPSS Statistics 26.0 statistical software, and results were regarded as significant at α = 0.05.
Results: Monomer release (triethylene glycol dimethacrylate, urethane dimethacrylate, and Bisphenol A glycerolate dimethacrylate) was significantly higher in the IC group. Mean cell viability was significantly lower in the HCB group than in the IC group.
Conclusion: All monomers in the tested materials were released at rates that were below clinical significance. Cell adhesion rates in the groups were similar. Cytotoxic response was classified as minor in the HCB and PR groups and non-cytotoxic in the IC group.
When there is excessive material loss in a posterior tooth, a direct composite restoration may not always provide adequate function and morphology. Direct composite resin restorations can be produced in a single session, are less expensive, and require no or minimal preparation of dental tissue. However, because indirect restorations are stronger, they support the remaining dental hard tissues and preserve the ideal anatomic form of the tooth [1]. Several recent studies have recommended bonded restorations rather than traditional fixed full crowns when substantial healthy dental tissue must be removed [2,3]. Partial restorations that are bound to the tooth with adhesive cement and restore one or more cusps on the whole occlusal surface and adjacent occlusal surfaces can be planned as inlay or onlay restorations [4].
Production techniques for indirect posterior restorations vary, as do the materials used for these techniques. Ceramic and composite resin materials are generally used in indirect posterior restorations, but computer-assisted design (CAD) and computer-aided manufacture (CAM) processes have resulted in notable advances in the production of ceramic and ceramic-like dental materials. However, because of the high cost and cervical margin incompatibility of the ceramic blocks used in indirect partial restorations, adaptation and aesthetic properties are provided by resin-based, hybrid nanoceramics [5].
Additive production technologies, known as 3-dimensional (3D) printing, and restorations using resin, a printable biomaterial, have recently come to the fore [6]. Printable resins that can be used to produce permanent restorations are now available. Crowns produced by 3D printing methods using permanent resin were reported to have satisfactory mechanical properties and high fracture resistance [7]. However, very few studies have examined monomer release and the potential cytotoxicity of such resins [8,9].
Because pure Bisphenol A (BPA) is toxic, BPA derivatives are used in dentistry [10]. Resin-containing restorative materials are produced with the addition of different amounts of monomers such as Bisphenol A glycerolate dimethacrylate (BisGMA), triethylene glycol dimethacrylate (TEGDMA), urethane dimethacrylate (UDMA), 2-hydroxyethyl methacrylate (HEMA), and Bisphenol A ethoxylate dimethacrylate (BisEMA) [11]. However, UDMA was recently replaced by BisGMA in several composite resins because of the latter’s low viscosity, high flexibility, high polymerization rate, high degree of transformation, and greater biocompatibility [12].
Residual monomers exposed to disruption because of insufficient polymerization and fluid in the oral environment adversely affect the system by impairing the mechanical and biological properties of the material [13]. Clinically safe materials must retain their biocompatibility in the intraoral environment. One measure of biocompatibility is the extent to which a material can sustain cell viability in its environment and with materials it contacts [14]. Although many studies have examined monomer release and the biocompatibility of direct resin composites [15,16,17], few have evaluated indirect resin composites/restorative materials [18].
This study evaluated monomer release, cell adhesion, and cell viability during a 24-h period of resin-containing indirect restorative materials produced by additive, subtractive, and conventional techniques. The null hypothesis was that residual monomer release, cell adhesion, and cell viability of 3D printable permanent resin, hybrid ceramic block, and indirect composite materials would be similar.
The present study and protocol were approved by the ethics committee of the Cukurova University Faculty of Medicine Clinical Research Ethics Committee (report numbered 2022/125.58). The properties of the three resin-containing indirect restorative materials used in this study are shown in Table 1.
| Material | Classification | Composition | Shade/ Lot number |
Manufacturer |
|---|---|---|---|---|
| PR | 3D printable permanent crown resin | esterification products of 4,40-isopropylidenediphenol, ethoxylated and 2-methylprop-2-enoic acid, diphenyl (2,4,6-trimethylbenzoyl) phosphine oxide (<2.50) | A2 / 600163 | Formlabs, Somerville, MA, USA |
| HCB | Hybrid ceramic block | urethane dimethacrylate, N, N-Dimethylacetamide, Polyoxyethyl-2,2-bis(p-hydroxyphenyl) propane dimethacrylate | A2 LT 14 / 2108021 | Cerasmart, GC, Tokyo, Japan |
| IC | Indirect composite | urethane dimethacrylate, 2,2-Dimethyl-1,3-propanediyl bismethacrylate, 1,3,5-Triazine-2,4,6-triamine, polymer with formaldehyde | HB-DA2 / 220311A | Gradia Plus, GC |
A total of 45 samples (5 for each experiment) of the RIR materials were prepared. To complete the polymerization stage, light-cured samples were analyzed after being stored in the dark for 24 h. To prepare the permanent resin (PR) samples, a file was prepared in standard tessellation language format, with dimensions of 8 × 8 × 2 mm3. Printable PR samples were prepared by using a layer thickness of 50 µm in the 3D printer (Formlabs, Somerville, MA, USA) with this file format. The samples were washed for 4 min with 99% pure isopropyl alcohol in a Form Wash device (Formlabs, Somerville). Then the first polymerization was applied for 20 min at 60°C in a Form Cure device (Formlabs, Somerville). After cutting the support sections of the samples, they were turned upside down and a second polymerization was applied for 20 min at 60°C.
HCB samples of 8 × 8 × 2 mm3 were prepared by cutting the CAD-CAM block (Cerasmart, GC, Tokyo, Japan) under water cooling and at low speed with a sensitive cutting device (Struers Accutom 10, Copenhagen, Denmark). To prepare the IC samples, teflon molds of appropriate size were used. The indirect composite samples (Gradia Plus, GC) were placed in a single layer in the teflon molds, compressed from above and below with strip bands, then produced with the same dimensions as samples in the other groups by polymerizing for 10 s in standard power mode (1000 mW/cm2) with a light device (VALO Ultradent, South Jordan, UT, USA) (step-mode). The samples were then removed from the teflon molds and cured again for 3 min with a Labolight DUO device (GC Europe, Leuven, Belgium), in accordance with the manufacturer’s instructions (full-mode).
Monomer releaseRelease of residual resin monomers from the prepared samples was evaluated after placing the samples in 5 mL of 75% ethanol/water solution in amber bottles incubated at 37°C. The fluid obtained from these solutions was placed in vials, and residual monomer release was analyzed in an HPLC-UV device (Shimadzu Nexera 2, Shimadzu Corporation, Kyoto, Japan). A 30:70 water/acetonitrile solution was used as the mobile phase in the analysis of the four resin monomers (TEGDMA, HEMA, UDMA, BisGMA).
The mobile phase was applied at a rate of 1.0 mL/min, a column temperature of 25°C, and with absorbance readings at 210 nm. The Inertsil ODS-4 C18 (250 mm × 4.6, 5 µm) was used as the column. The volume of fluid injected to the column from each sample was 20 µL.
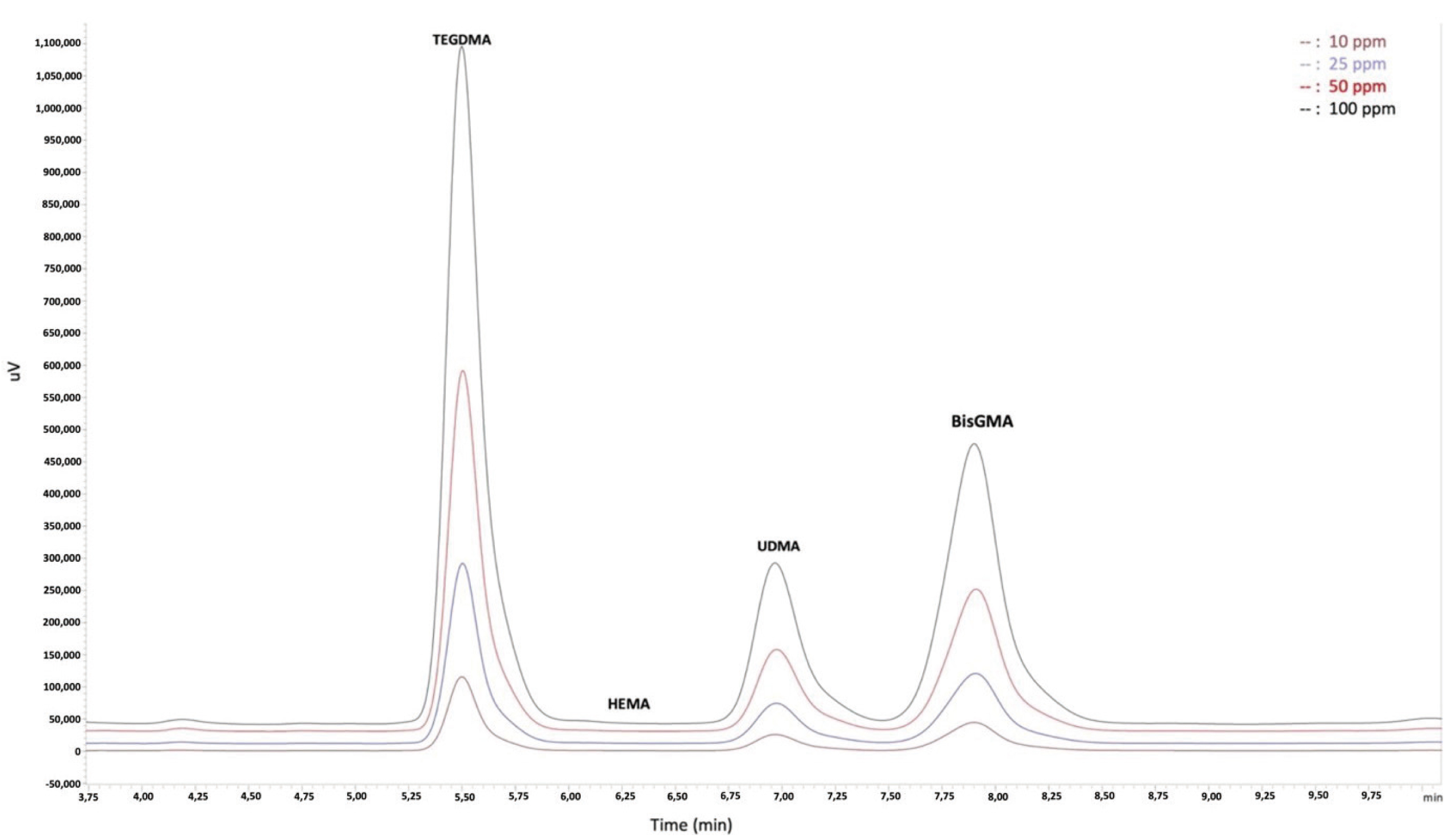
To determine residual monomer concentrations, the calibration curves of the resin monomers were created (Fig. 1). Two different calibration curves were formed for each monomer in the concentration ranges of “10, 25, 50, and 100 ppm” and “0.5, 1, 2.5, and 5 ppm”. The calibration range for the HEMA monomer is 10, 25, 50, 100 and 150 ppm. Because all HCB samples remained outside the defined calibration range for TEGDMA concentration, it was calculated by using single-point calibration. Some PR samples remained outside the defined calibration range for HEMA concentration, so it was calculated by using single-point calibration.
Direct and indirect cytotoxicity studies were conducted in accordance with ISO-10993 standard guidelines [19]. L929 rat fibroblast cell line (CRL-2097, ATCC, USA) was cultured in low glucose Dulbecco’s Modified Eagle Medium (L-DMEM) (GIBCO) containing 10% fetal bovine serum (FBS) (GIBCO) and 1% penicillin/streptomycin (pen/strep) at 37°C, 5% CO2 atmosphere, and 95% relative humidity. When the cells reached 80% confluence, they were trypsinized and counted to determine cell viability. Cell adhesion and cell viability in this study were determined with water-soluble tetrazolium salt (WST-1) analysis. In both studies, the control group was medium containing no material, but all other conditions were identical, and the same number of cells was seeded in the prepared control medium.
Cell adhesionTo analyze cell adhesion to the restorative materials, both surfaces of the samples were sterilized for 15 min in a laminar flow cabinet under UV light in sterile conditions, after which the samples were placed in 24-well culture dishes. On each sample, 2 × 104 cells were seeded, and these cells were cultured in L-DMEM medium containing 1% pen/strep and 10% FBS for 24 h at 37°C in a 5% CO2 atmosphere. To separate cells adhering to the material at the end of incubation, the materials were washed with phosphate buffered saline and then placed in a new 24-well culture dish not containing cells. L-DMEM containing 10% WST-1 reagent (Roche) was added to cover the samples, which were then incubated in the dark for 2 h at 37°C and 5% CO2. As a blank, L-DMEM containing 10% WST-1 reagent but no cells or material was used. At the end of incubation, measurements were recorded with a spectrophotometer (VerseMax) at a wavelength of 450 nm and then analyzed.
Cell viabilityTo prepare extracts of the ISO 10993-12 test materials [19], the extraction rate is 3 cm2/mL for materials such as synthetic polymer, ceramic, and composites thicker than 0.5 mm (expressed as the ratio [±10%] of the extract used of the surface area or mass of the test sample to the volume). Thus, 0.64 mL of extraction fluid was used for one test sample measuring 8 × 8 × 2 mm3 and with a surface area of 192 mm2. The prepared samples (0.64 mL × 5) in 3.2 mL of medium (L-DMEM culture medium containing 1% pen/strep and 10% FBS) were placed in sterile tubes, and the eluate was then prepared by incubating the samples for 24 h at 37°C in 5% CO2. With the addition of 2 × 104 cells to a 96-well plate, they were then cultured for 24 h at 37°C in 5% CO2.
WST analysisAt the end of 24 h of incubation, the medium in the wells was replaced with L-DMEM containing 10% WST-1 reagent (Roche), after which the cells were incubated in the dark for 2 h at 37°C in an environment containing 5% CO2. As a blank, L-DMEM containing 10% WST-1 reagent, but no cells or material, was used. At the end of incubation, measurements were recorded with a spectrophotometer (VerseMax) at a wavelength of 450 nm and then analyzed.
Statistical analysisData obtained in the study were analyzed by using IBM SPSS Statistics version 26.0 (IBM SPSS, Armonk, NY, USA). The data distribution was assessed with the Shapiro–Wilk test, and the Levene test was used to determine homoscedasticity. One-way ANOVA was used to evaluate differences between three or more independent groups of data with normal distributions. The Bonferroni test was used for post-hoc comparisons of variables that were significant in ANOVA. Comparisons between two independent groups of data with normal distributions were made with the independent samples t-test. Associations between numerical variables were evaluated with Pearson correlation analysis. Descriptive statistics are expressed as mean ± SD. Statistical significance was defined as α = 0.05.
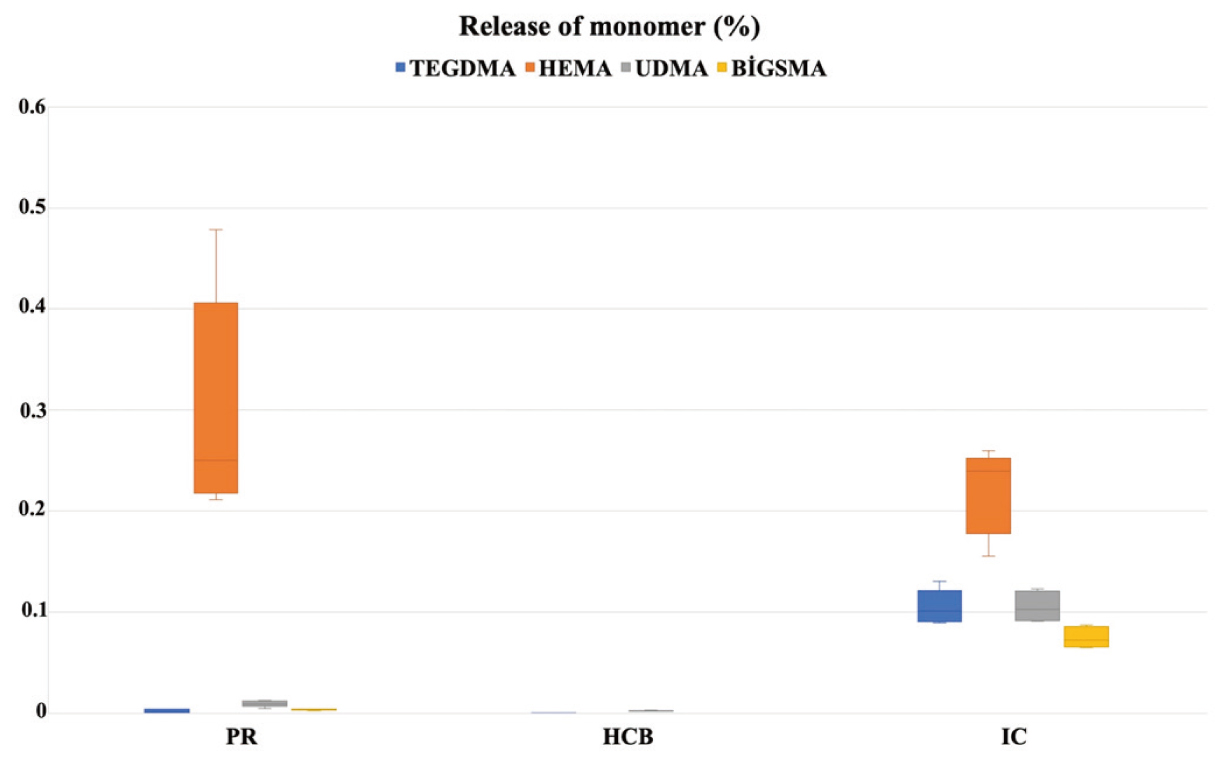
Monomer release for the PR, HCB, and IC groups is expressed as concentrations (ppm) in Table 2 and as percentages (%) in Figure 2. The Levene test showed equality of variances (TEGDMA P = 0.158 [ppm] and P = 0.059 [%]; HEMA P = 0.314 [ppm] and P = 0.089 [%]; UDMA P = 0.072 [ppm] and P = 0.103 [%]; BisGMA P = 0.066 [ppm] and P = 0.322 [%]). Monomer release significantly differed between the TEGDMA, HEMA, UDMA, and BisGMA groups (P < 0.001). In post-hoc comparisons, TEGDMA and UDMA release was significantly higher in the IC group than in the other groups (P < 0.001). HEMA release did not significantly differ between the PR and IC groups (P = 0.635). BisGMA release was undetectable in the HCB group and significantly higher in the IC group than in the PR group (P < 0.001).
| PR1 | HCB2 | IC3 | P*$-value | Post-hoc P-value | |
|---|---|---|---|---|---|
| TEGDMA | 0.843 ± 0.69 | 0.092 ± 0.02 | 59.478 ± 8.68 | <0.001* | 1-2: 1 1-3: <0.001 2-3: <0.001 |
| HEMA | 114.17 ± 43.34 | NA | 125.290 ± 25.85 | 0.635$ | - |
| UDMA | 3.580 ± 1.21 | 1.350 ± 0.27 | 59.795 ± 7.55 | <0.001* | 1-2: 1 1-3: <0.001 2-3: <0.001 |
| BisGMA | 1.391 ± 0.27 | NA | 42.436 ± 5.17 | <0.001$ | - |
*One-way ANOVA. $Independent sample t test. Data are expressed as mean ± SD. Post-hoc comparisons were made with the Bonferroni test.
The cell adhesion values of the PR, HCB, IC, and control groups are shown in Table 3. The Levene test showed equality of variances (P = 0.654). Post-hoc comparisons showed that the cell adhesion values were significantly higher in the control group than in the experimental groups (P < 0.001). In paired comparisons of the experimental groups, cell adhesion values were similar (P > 0.05).
| PR1 | HCB2 | IC3 | C4 | P*-value | Post-hoc P-value | |
|---|---|---|---|---|---|---|
| Cell adhesion | 3,795 ± 187.73 | 3,718 ± 55.47 | 3,751 ± 133.87 | 21,047 ± 2,617.81 | <0.001 | 1-2: 1 2-3: 1 1-4: <0.001 2-4: <0.001 1-3: 1 3-4: <0.001 |
| Cell viability | 20,969 ± 3,907.61 | 19,055 ± 3,187.57 | 25,593 ± 2,223.49 | 21,426 ± 3,499.04 | 0.038 | 1-2: 1 2-3: 0.036 1-4:1 2-4:1 1-3: 0.238 3-4: 0.365 |
*One-way ANOVA. Data are expressed as mean ± SD. Post-hoc comparisons were made with the Bonferroni test.
Cell viability
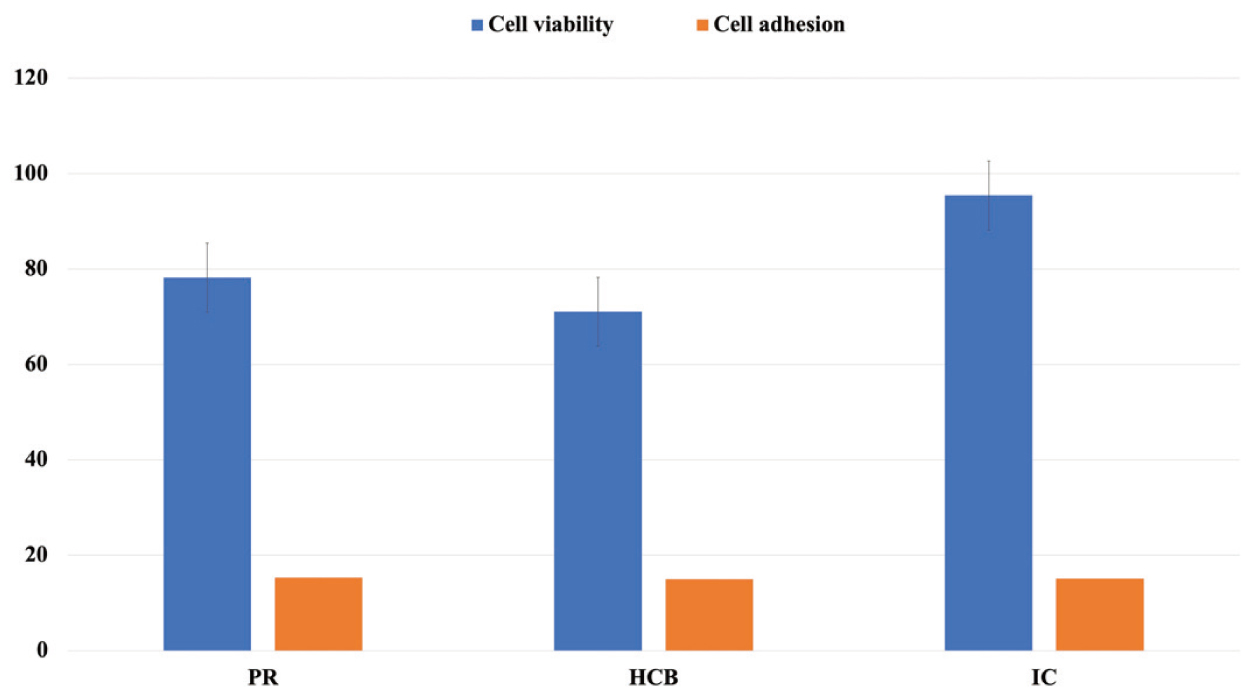
The cell viability values of the PR, HCB, IC, and control groups are shown in Table 3. The Levene test showed equality of variances (P = 0.731). Mean cell viability was significantly lower in the HCB group than in the IC group (P = 0.036). The mean survival rates of cells in direct and indirect contact after 24 h were compared between groups by setting the highest cell viability value to 100% (Fig. 3). The cytotoxic response to a dental material was graded as non-cytotoxic (>90% survival), minor (60-90% survival), moderate (30-60% survival), or severe (<30% survival) [20]. The cytotoxic responses to HCB and PR were minor, at 71% and 78% respectively, and the IC group was non-cytotoxic, at 95%.
The results of statistical analyses of the associations between monomer release, cell adhesion, and cell viability (as ppm and %) are shown in Table 4. There was no significant association between cell viability and cell adhesion or between cell adhesion and monomer release (ppm and %) (P = 0.972). Among the monomer release variables, a moderate positive level correlation was seen between TEGDMA, UDMA, and BisGMA, and cell viability (P < 0.05). Cell viability and HEMA monomer release were not significantly associated (P = 0.967, P = 0.245).
The results of analysis of associations between monomer release, cell adhesion, and cell viability values (ppm and %) in the three material groups are shown in Table 5. Cell viability and monomer release were not significantly associated in the PR group (P > 0.05), although there was a significant positive, low-level association between cell adhesion and UDMA monomer release (r = 0.070, P = 0.041; r = 0.092, P = 0.023). There was no significant correlation of cell adhesion with the other monomer release values (P > 0.05).
| Cell adhesion | Cell viability | |||
|---|---|---|---|---|
| r | P- value | r | P- value | |
| Cell adhesion | 0.090 | 0.972 | 0.090 | 0.972 |
| Release of monomer | ||||
| TEGDMA (ppm) | −0.010 | 0.973 | 0.671 | 0.006 |
| HEMA (ppm) | 0.574 | 0.083 | 0.015 | 0.967 |
| UDMA (ppm) | −0.012 | 0.966 | 0.679 | 0.005 |
| BisGMA (ppm) | −0.160 | 0.659 | 0.653 | 0.041 |
| TEGDMA (%) | −0.041 | 0.884 | 0.666 | 0.007 |
| HEMA (%) | 0.605 | 0.064 | −0.405 | 0.245 |
| UDMA (%) | −0.033 | 0.908 | 0.674 | 0.006 |
| BisGMA (%) | −0.201 | 0.578 | 0.643 | 0.045 |
r: Pearson correlation coefficient
| Cell adhesion | Cell viability | |||
|---|---|---|---|---|
| PR | r | P-value | r | P-value |
| Cell adhesion | −0.406 | 0.498 | −0.406 | 0.498 |
| Release of monomer | ||||
| TEGDMA (ppm) | −0.154 | 0.805 | 0.151 | 0.808 |
| HEMA (ppm) | 0.845 | 0.071 | −0.271 | 0.659 |
| UDMA (ppm) | 0.895 | 0.040 | −0.468 | 0.427 |
| BisGMA (ppm) | 0.543 | 0.344 | 0.049 | 0.937 |
| TEGDMA (%) | −0.129 | 0.837 | 0.142 | 0.820 |
| HEMA (%) | 0.864 | 0.059 | −0.257 | 0.677 |
| UDMA (%) | 0.092 | 0.023 | −0.452 | 0.445 |
| BisGMA (%) | 0.594 | 0.291 | 0.105 | 0.866 |
| HCB | r | P-value | r | P-value |
| Cell adhesion | 0.274 | 0.656 | 0.274 | 0.656 |
| Release of monomer | ||||
| TEGDMA (ppm) | 0.750 | 0.144 | 0.342 | 0.573 |
| HEMA (ppm) | NA | NA | NA | NA |
| UDMA (ppm) | −0.558 | 0.328 | 0.444 | 0.454 |
| BisGMA (ppm) | NA | NA | NA | NA |
| TEGDMA (%) | 0.627 | 0.258 | 0.617 | 0.267 |
| HEMA (%) | NA | NA | NA | NA |
| UDMA (%) | −0.603 | 0.281 | 0.359 | 0.553 |
| BisGMA (%) | NA | NA | NA | NA |
| IC | r | P-value | r | P-value |
| Cell adhesion | −0.537 | 0.351 | −0.537 | 0.351 |
| Release of monomer | ||||
| TEGDMA (ppm) | 0.129 | 0.837 | 0.243 | 0.694 |
| HEMA (ppm) | 0.084 | 0.893 | 0.319 | 0.601 |
| UDMA (ppm) | −0.170 | 0.785 | 0.457 | 0.439 |
| BisGMA (ppm) | −0.173 | 0.780 | 0.493 | 0.399 |
| TEGDMA (%) | −0.223 | 0.718 | 0.117 | 0.851 |
| HEMA (%) | −0.129 | 0.723 | 0.280 | 0.649 |
| UDMA (%) | −0.553 | 0.333 | 0.295 | 0.630 |
| BisGMA (%) | −0.573 | 0.313 | 0.317 | 0.604 |
r: Pearson correlation coefficient
The most recent research strongly recommends resin-containing indirect materials for partial restorations [21]. However, this recommendation is based on the general mechanical and physical properties of these materials [22]. Safe intraoral use of resin-containing materials requires minimal residual monomer release and maximum biocompatibility with cells in the environment and with which they are in contact.
The results of this study of the biological effects of three resin-containing indirect restorative materials demonstrated that TEGDMA, UDMA, and BisGMA monomer release was significantly greater in the IC group than in the HCB and PR groups, but because this effect remained below the level of clinical significance, cell viability and cell adhesion were unaffected. Cell adhesion was similar in the present materials. IC was non-toxic, whereas HCB and PR exhibited minor cytotoxicity. Therefore, the null hypothesis of the study was rejected.
HPLC analysis was used to identify monomer release in this study, as it is better than other methods in evaluating high-molecular-weight monomers such as BisGMA and UDMA [23]. To determine if monomer release is clinically significant, the US Food and Drug Administration recommends the use of a 75% ethanol-water solution as food/mouth simulation fluid, as in this study [24]. There are various in-vitro cytotoxicity tests available, and the MTT test [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] is often used to determine cell viability. However, the WST-1 test, which is three times as sensitive as the MTT test, was used in this study [25].
Resin-containing CAD-CAM materials are preferred to indirect restorations because, as compared with glass-ceramics, they are less fragile, have greater polishability and marginal integrity, and cause less wear in the opposite teeth [21]. In recent years, 3D printable permanent resins have started to be used for construction of indirect restorations.
In a study by Graf et al. [26], the bonding properties of a 3D printable permanent material (BEGO VarseoSmile Crown plus) were compared with those of milled composite materials (Vita Enamic ve 3M Lava Ultimate) in partial restorations. Analysis of the pull-off forces of the materials confirmed that the bonding values were sufficiently high for clinical use. Therefore, resin-containing indirect restorations, which are now used more frequently in clinics, are preferred for teeth with greater material loss. However, their surface area in the mouth is greater than that of composite resin restorations applied with the direct method. This suggests that there could be greater monomer release in these materials and that the cytotoxic effect could be increased. However, this potential drawback can be addressed by completing polymerization outside the mouth or with high temperature/pressure production, or by using additional procedures to remove residual monomers after polymerization.
The most important problem affecting biocompatibility in resin-containing materials is the presence of residual monomers after polymerization. Although residue percentage has decreased in the last 10 years, it remains a problem. The amount of residual monomer should be less than 10% of the remaining methacrylic groups and is estimated to be a maximum of 1.5-5% [27]. Release from dental composites of materials such as BPA, which is toxic, is a matter of concern [28].
In the current study, BisGMA release was undetectable in the HCB group, minimal in the PR group (1.391 ± 0.27 ppm), and small for IC, which is a UDMA-based composite (42.436 ± 5.17 ppm). BisGMA release did not exceed 0.1% in any group. Among the monomers tested in this study, excepting HEMA (0.2-0.3%), the mean release of monomers did not exceed 0.1%. Thus, monomer release of the materials tested did not exceed the threshold of clinical significance [27] and no serious cytotoxic potential was noted.
The cytotoxic effects of resin monomers, generally ranked as BisGMA > UDMA > TEGDMA > HEMA, depend on the monomer component of the materials [29]. HEMA is a low-molecular-weight monomer that is an important component of most adhesive systems and is characterized by hydrophilic properties [30]. While HEMA monomer was not observed in the HCB group, release in the range of 0.2-0.3% was seen in the PR and IC groups. However, there was no negative effect on cell viability or cell adhesion. Unlike BisGMA, UDMA does not contain aromatic groups and has a lower molecular weight and viscosity. This increases the resistance of the material, and decreased monomer release increases cell viability [31]. The fact that the three present materials had a high weighting of UDMA monomer explains their limited cytotoxicity.
The hydrophilic monomers HEMA and TEGDMA can spread from the dentin to the pulp chamber at high concentrations [32]. As compared with BisGMA, TEGDMA has greater solubility, resulting in greater leakage into the environment. However, the combination of BisGMA and TEGDMA results in a high degree of transformation, with less solubility and absorption in water [33]. Previous reports indicate that resins containing BisGMA and TEGDMA have extremely stable acrylic bonds, thereby forming a 3-dimensional network with good mechanical and chemical properties [34]. Despite the BisGMA content of the present IC group, cell viability was greater than in the other groups because of the combination with TEGDMA. Because of the absence of the secondary functional (−OH) group, Bis-EMA is an ethoxylated analog of BisGMA. Like BisGMA, it contains BPA, which is toxic [27]. Information from the manufacturer suggests that the lower cell viability in the HCB group may be due to the BisEMA content.
To the present authors’ knowledge, no previous study evaluated residual monomer release of the permanent resin (Formlabs) used in the PR group of this study, which is an important strength of this study. In a study [19] of residual monomer release of Gradia Plus from an IC group, UDMA release was 1.364 ± 0.99 µg/mL for the crown section (surface area = 3.15 cm2) of a first molar made with Gradia Plus. Neither BisGMA nor BPA were detected. In the present study, TEGDMA release from Gradia Plus was 59.478 ± 8.68 ppm, UDMA release was 59.795 ± 7.55 ppm, and BisGMA release was 42.436 ± 5.17 ppm. This difference can be attributed to the environment in which the samples were dissolved: HPLC-grade water was used for this purpose by Hampe et al. [18], whereas a 75% ethanol-water solution was used in the present study, as it best simulates the oral environment.
Two previous studies assessed residual monomer release in Cerasmart, which was included in the HCB group. In a study by Putzeys et al. [35], UDMA release was small in a Cerasmart group throughout the 8 weeks of the experiment (cumulative release: 1.24 ± 0.06 nmol), and this result was similar to that of the current study (at the end of 24 h: 1.35 ± 0.27 nmol). The same study reported that monomer release was lower in indirect restorations than in cemented indirect and direct restorations. Therefore, optimal placement of indirect restorations and use of the thinnest possible layer of cement were recommended.
Barutcigil et al. [36] reported UDMA release from Cerasmart of 5.73 ± 0.61 µg/mL after 24 h and 6.20 ± 0.16 µg/mL after 3 months. BisEMA release was 6.98 ± 0.81 µg/mL after 3 months, and no HEMA, TEGDMA, or BisGMA release was reported. Similar results were obtained in the current study. UDMA release from Cerasmart was 1.35 ± 0.27, TEGDMA release was 0.092 ± 0.02, and no HEMA or BisGMA monomer release was detected.
No previous cytotoxicity study examined Formlabs Permanent Resin or Gradia Plus of the present IC group. This is another strength of the present study. Three previous studies investigated Cerasmart, which was used in the HCB group, and the present results are consistent with previously reported findings [37,38,39].
Hassan et al. [38] evaluated the effect on cell viability of three CAD-CAM blocks, namely two resin-based composites (Brilliant Crios-BC and Cerasmart-CS) and a hybrid ceramic (Vita Enamic-EN). The greater viability of CS and EN, as compared with BC, might have been due to the UDMA content rather than to BisGMA. This result regarding UDMA monomer content might explain the limited toxicity of the present materials.
In contrast to the present results, a study [37] of the effects of novel full ceramic and temporary materials on the L929 cell line found that, among the ceramic material groups, cell viability was greater in the Cerasmart group in medium extract measurements on the first day. Ille et al. [39] reported that in EDX spectrum analysis Cerasmart restorative material released four main inorganic components into the cell culture environment: silicon, barium, aluminum, and fluorine. In the current study, the fact that Cerasmart was more cytotoxic than the other materials can be explained by the presence of barium, which is present at a high percentage by weight and is known to be toxic [40]. Although resin monomer release in Cerasmart was lower than in the other groups, the greater cytotoxicity is likely attributable to the inorganic content.
In conclusion, the present results demonstrate that residual monomer release from the indirect resin-containing restorative materials HCB, PR, and IC was not clinically significant, that cells could adhere to the materials, and that the limited cytotoxicity of the materials indicated clinical biocompatibility. Future animal and clinical studies should evaluate additional residual monomers in dental materials.
The authors declare no conflicts of interest in relation to this work.
This study was supported by the Cukurova University Scientific Research Projects Coordination Unit (Project no: TSA-2022-15486).
The authors would like to thank Prof. Dr. Yurdanur UCAR for a critical review of this study.