Abstract
Purpose: Alveolar osteitis (dry sockets) is a painful condition characterized by a limited immune response. It is typically caused by the removal of blood clots from extracted tooth sockets, which leads to the fermentation of trapped food remnants by oral bacteria in the cavities, producing high concentrations of short-chain fatty acids (SCFAs). This study examined the effects of SCFAs on immunity and bone metabolism.
Methods: Mouse macrophage Raw264.7 cells were treated with oral bacteria supernatants or SCFA mixtures, and inducible nitric oxide synthase (iNOS) levels were determined by western blot. The same cells were treated with SCFA mixtures in the presence of receptor activator of nuclear factor-kappa B ligand (RANKL), and osteoclast-like cells were counted. MC3T3-E1 cells were treated with SCFA mixtures and stained with alizarin red S.
Results: Raw264.7 cells treated with oral bacterial culture supernatants of Porphyromonas gingivalis and Fusobacterium nucleatum inhibited lipopolysaccharide (LPS)-induced iNOS production, likely due to SCFA content. SCFA mixtures mimicking these supernatants inhibited the number of RANKL-induced tartrate-resistant acid phosphatase (TRAP)-positive cells and MC3T3-E1 cell mineralization.
Conclusion: These data suggest that SCFAs produced by P. gingivalis and F. nucleatum may reduce the inflammatory response and mildly induce mineralization of the alveolar walls. These results may contribute to the understanding of alveolar osteitis.
Introduction
Alveolar osteitis is a common complication after tooth extraction and is often referred to as dry socket. When blood clots are removed from the tooth sockets following dental extraction, alveolar osteitis reportedly occurs at an incidence rate of 0.5–5% for routine extractions and more than 30% for third molar extractions of the mandible [1,2]. Typical symptoms of the condition include intense radiating pain, which is challenging to alleviate with analgesia, in or around the extraction socket, along with halitosis. These symptoms cause discomfort for patients following tooth extraction. Although this condition has been known for many years, the etiology of alveolar osteitis is not well understood. During the wound healing process of an extraction socket, blood clots are created in the cavities, leading to the formation of granulation tissue, which is characterized by the presence of numerous blood capillaries, and following bone formation occurs within the cavities. When the blood clots are completely absent or lysed soon after small clot formation, underlying cavity bone surfaces are directly exposed to the oral cavities. These conditions result from plasmin activation that lyse fibrin nets in the blood clots. This is the widely accepted etiology of alveolar osteitis which is referred to as the fibrinolytic theory [1,3]. Since the fibrinolytic activities are also induced by plasmin activation by bacteria, poor oral hygiene can be one of the most important risk factors of alveolar osteitis.
There are several hundred species of microorganisms that inhabit the oral cavity, some of which have inflammation activators such as lipopolysaccharides (LPS). Although these bacteria can flow into alveolar cavities, marked signs of inflammation by bacterial infection such as fever onset and lymphadenopathy are rarely observed in alveolar osteitis [4,5,6]. The reason for this phenomenon remains unclear. Removing blood clots from the alveolar sockets following tooth extraction creates residences on the socket’s surfaces for bacteria to multiply. Impacted food remnants in the cavities are fermented and metabolites are produced by bacteria. Macrophages, patrolling around the surfaces of the socket, encounter these bacterial metabolites. The effect of bacterial culture supernatants from different species on LPS-induced macrophage activation was first investigated in this study. Since the supernatants contain high concentrations of short-chain fatty acids (SCFAs), the effects of SCFAs on LPS-induced macrophage activation, and their influence on receptor activator of nuclear factor-kappa B ligand (RANKL)-induced osteoclast differentiation and osteoblast bone formation were also investigated. The roles of bacterial metabolites for the development of alveolar osteitis were also discussed.
Materials and Methods
Reagents
Sodium salts of butyrate, propionate, acetate, formate, and disodium succinate, disodium β-glycerophosphate pentahydrate, and L-ascorbic acid phosphate magnesium salt n-hydrate were purchased from Fujifilm Wako (Osaka, Japan). Sodium isobutyrate and sodium isovalerate were purchased from Kanto Chemical (Tokyo, Japan) and sodium lactate from Sigma Aldrich (St. Louis, MO, USA).
Oral bacteria used in this study, culture condition, and bacterial cellular supernatant preparations
Oral bacteria including Porphyromonas gingivalis (P. gingivalis) strains W83 and FDC381, Fusobacterium nucleatum (F. nucleatum) strains ATCC25586 and JCM6328, Prevotella intermedia (P. intermedia) strain ATCC25611, Prevotella nigrescens (P. nigrescens) strain ATCC33563, and Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) strain Y4 were anaerobically cultured in brain heart infusion (BHI) broth (BD, Franklin Lakes, NJ, USA) supplemented with 5 ppm hemin and 0.5 ppm menadione at 37°C, and cell culture supernatants were collected by centrifugation at 10,000 × g for 20 min at 4°C to remove bacterial cells and sterilized using 0.22-µm pore size filter units. One tenth of the bacterial culture supernatants were diluted in mammalian cell culture medium.
SCFA quantitation in bacterial culture supernatants and preparation of SCFA mixtures which mimics bacterial culture supernatants
SCFA concentrations in the bacterial culture supernatants were determined using gas chromatography as previously described [7]. Briefly, phosphoric acid-acidified bacterial culture supernatants were mixed with two times volume of acetonitrile. After protein or peptide precipitation by centrifugation, the supernatants were applied to a GC-2010 capillary gas chromatograph (Shimadzu, Kyoto, Japan) with a free fatty acid phase capillary column. SCFA-mixtures, designed to mimic the composition found in bacterial cell culture supernatants, were prepared based on the SCFA concentrations observed in each bacterial culture supernatant.
Cell culture
Mouse macrophage Raw264.7 cells were purchased from Dainippon Pharmaceutical (Osaka, Japan). MC3T3-E1 cells was provided by the Riken BioResource Research Center (Tsukuba, Japan). Both cell lines were maintained in 10% fetal bovine serum (FBS, Cytiva, Marlborough, MA, USA) containing minimum essential medium α (MEMα, Fujifilm Wako) supplemented with penicillin and streptomycin (Fujifilm Wako) at 37°C. For mineralization experiments, 10% FBS MEMα containing 10 mM disodium β-glycerophosphate pentahydrate and 50 µG/mL L-ascorbic acid phosphate magnesium salt n-hydrate was used as a mineralization medium.
Western blot
Raw264.7 cells (4 × 106 cells/well) were plated onto a 24-well cell culture plate. Cells were treated for 8 h with bacterial culture supernatants (1/10 volume), sodium butyrate (5 or 10 mM), sodium propionate (10 or 20 mM), sodium isobutyrate (10 or 20 mM), sodium isovalerate (10 or 20 mM), sodium acetate (10 or 20 mM), sodium lactate (10 or 20 mM), sodium formate (10 or 20 mM), disodium succinate (10 or 20 mM), or mimics of bacterial culture supernatants. Equal amounts of total protein from cell lysates were applied to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (5-20% polyacrylamide gradient gel), and the separated proteins were transferred onto a polyvinylidene difluoride membrane. The membrane was treated for 30 min with an Ez-Block Chemi blocking reagent (ATTO, Tokyo, Japan). An anti-inducible nitric oxide synthase (iNOS) polyclonal antibody (GeneTex, Irvine, CA, USA) and WestVision Peroxidase Polymer, anti-rabbit IgG (Vector Laboratories, Newark, CA, USA) were used as primary and secondary antibodies, respectively. An anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mouse IgG (Santa Cruz Biotechnology, Dallas, TX, USA) and WestVision Peroxidase Polymer, anti-mouse IgG (Vector Laboratories) were used for internal control detection. The band images were developed using a Clarity Western ECL reagent (BIO-RAD, Hercules, CA, USA) and photographed using a ChemiDoc XRS system (BIO-RAD).
Osteoclast-like cell differentiation
Raw264.7 cells (3 × 103 cells/well) were plated on a 48-well plate and the plate was incubated overnight at 37°C. Media were changed to 10% FBS MEMα containing sodium butyrate (1 mM), sodium propionate (5 mM), sodium isobutyrate (10 mM), sodium isovalerate (10 mM), sodium acetate (10 mM), sodium lactate (10 mM), sodium formate (10 mM), disodium succinate (10 mM), or SCFA mixtures that mimic bacterial culture supernatants. Recombinant mouse RANKL (PeproTech, Cranbury, NJ, USA) at a concentration of 50 nG/mL was added, and cells were cultured for 4 days. Cells were fixed by 10% formaldehyde (Fujifilm Wako), treated with 0.1% Triton X-100, and stained with tartrate-resistant acid phosphatase (TRAP) staining solution. The plates were scanned with a GT-X830 flat-head scanner (Seiko Epson, Suwa, Japan). TRAP-positive cells that have more than three nuclei were counted as osteoclast-like cells.
Mineralization assay
A murine osteoblast-like MC3T3-E1 line is a group of cells with heterogeneous characteristics. Thus, using a limited dilution method, a clonal line was established and named clone NDC10E1, which can effectively form mineralized tissue. The clone forms mineralized tissue within 7 days in the mineralization medium and was used as preosteoblastic cells in this study. MC3T3-E1 clone NDC10E1 cells (2 × 105 cells/well) were plated on a 24-well plate and incubated overnight at 37°C (this condition can allow the cells to reach confluence the next day). Media were changed to a mineralization medium in the presence or absence of each SCFA or mimics of bacterial culture supernatants and incubated for 6 days at 37°C. Media were changed every 2 or 3 days. Alizarin red S staining was performed to evaluate mineralization. The stained plates were scanned using a GT-X830 (Seiko Epson) and mean red intensities of each well were measured using a Fiji/ImageJ software (https://imagej.net/software/fiji/).
Statistical analysis
Statistical analysis was conducted utilizing the EZR program provided by the Saitama Medical Center, Jichi Medical University, Saitama, Japan [8]. The Shapiro-Wilk normality test was employed to assess the distribution of the data, which revealed a non-normal distribution. Consequently, the Kruskal-Wallis test, followed by the Steel test, was utilized to evaluate the differences. Results yielding a P-value of less than 0.05 were interpreted as statistically significant.
Results
SCFAs produced by P. gingivalis and F. nucleatum suppress LPS-induced activation of M1 macrophages
To examine the effects of oral bacterial metabolites on LPS-induced macrophage activation, Raw264.7 cells were treated with culture supernatants of oral bacteria. Although LPS (100 nG/mL) treatment with Raw264.7 cells induces iNOS production, treatments with bacterial culture supernatants of P. gingivalis or F. nucleatum strongly suppressed LPS-induced iNOS production (Fig. 1A). On the other hand, supernatants from P. intermedia, P. nigrescens, and A. actinomycetemcomitans did not exhibit strong suppressions of LPS-induced iNOS levels. Since oral bacteria ferment dietary fibers and produce high concentrations (mM-order) of several types of SCFAs [9], each SCFA concentration in bacterial culture supernatants was examined using gas chromatography (Table 1). Relatively high concentrations of butyrate (19.095-32.414 mM), propionate (5.576-9.065 mM), and isobutyrate (8.156-19.991 mM) were found in culture supernatants of P. gingivalis and F. nucleatum of which culture supernatants strongly suppressed LPS-induced iNOS production (Table 1 and Fig. 1A). Next, the effects of each SCFA contained in bacterial culture supernatants on LPS-induced M1 macrophage activation were examined. Treatment of Raw264.7 macrophage cells with butyrate, propionate, isobutyrate, and isovalerate strongly suppressed LPS-induced iNOS production (Fig. 1B). Although acetate treatment slightly repressed LPS-induced iNOS production, other SCFA (lactate, formate, succinate) treatments did not reduce LPS-induced iNOS production (Fig. 1B). SCFA mixtures mimicking the respective bacterial culture supernatants were subsequently prepared and the effects of these mixtures on LPS-induced iNOS production were examined. Only SCFA mixtures that mimic P. gingivalis and F. nucleatum culture supernatant reduced LPS-induced iNOS production (Fig. 1C). These results demonstrated that SCFAs which are produced by P. gingivalis and F. nucleatum suppress LPS-induced macrophage activation. Especially, butyrate, propionate, isobutyrate, and isovalerate may have potent effects on this phenomenon.

Table 1 SCFA concentrations in supernatant of bacterial cultures
| Bacterial strains |
SCFA (mM) |
| Butyrate |
Propipnate |
Isobutyrate |
Isovalerate |
Acetate |
Formate |
Lactate |
Succinate |
| BHI broth containing |
|
|
|
|
|
|
|
|
| hemine and menadione |
0.000 |
3.637 |
4.837 |
0.000 |
2.875 |
0.284 |
0.532 |
0.233 |
| Porphyromonas gingivalis |
|
|
|
|
|
|
|
|
| W83 |
19.095 |
6.719 |
19.991 |
8.067 |
10.961 |
0.329 |
0.000 |
0.202 |
| FDC381 |
24.386 |
9.065 |
15.841 |
13.159 |
11.802 |
0.641 |
0.000 |
0.963 |
| Fusobacterium nucleatum |
|
|
|
|
|
|
|
|
| ATCC25586 |
32.414 |
7.365 |
8.219 |
0.000 |
11.452 |
8.824 |
0.000 |
0.202 |
| JCM6328 |
31.622 |
5.576 |
8.156 |
0.000 |
10.976 |
8.781 |
0.000 |
0.224 |
| Prevotella intermedia |
|
|
|
|
|
|
|
|
| ATCC25611 |
0.000 |
3.729 |
0.000 |
1.755 |
24.065 |
15.68 |
0.000 |
6.996 |
| Prevotella nigrescens |
|
|
|
|
|
|
|
|
| ATCC33563 |
0.000 |
3.464 |
0.000 |
2.737 |
17.183 |
8.337 |
0.000 |
8.675 |
| Aggregatibacter actinomycetemcomitans |
|
|
|
|
|
|
|
|
| Y4 |
0.000 |
3.446 |
0.000 |
0.000 |
15.598 |
2.96 |
9.789 |
2.894 |
SCFAs produced by oral bacteria inhibit RANKL-induced osteoclast formation
The effects of SCFAs produced by oral bacteria on bone metabolisms were subsequently examined. As shown in Fig. 2A, treatment with butyrate, propionate, isobutyrate, and isovalerate almost completely inhibited RANKL-induced TRAP-positive multinuclear cell formation. Although acetate, lactate, and succinate treatment significantly reduced RANKL-induced TRAP-positive multinuclear cell formation, more than 50 TRAP-positive cells were still observed (Fig. 2A). Although treatment with SCFA mixtures which mimic P. gingivalis or F. nucleatum culture supernatants almost completely suppressed RANKL-induced TRAP-positive multinuclear cell formation (Fig. 2B), treatment with SCFA mixtures which mimic culture supernatants of P. intermedia, P. negrescens, and A. actinomycetemcomitans significantly reduced RANKL-induced TRAP-positive cell formation (Fig. 2B). However, the reduced rates are weaker than those of P. gingivalis and F. nucleatum (Fig. 2B).

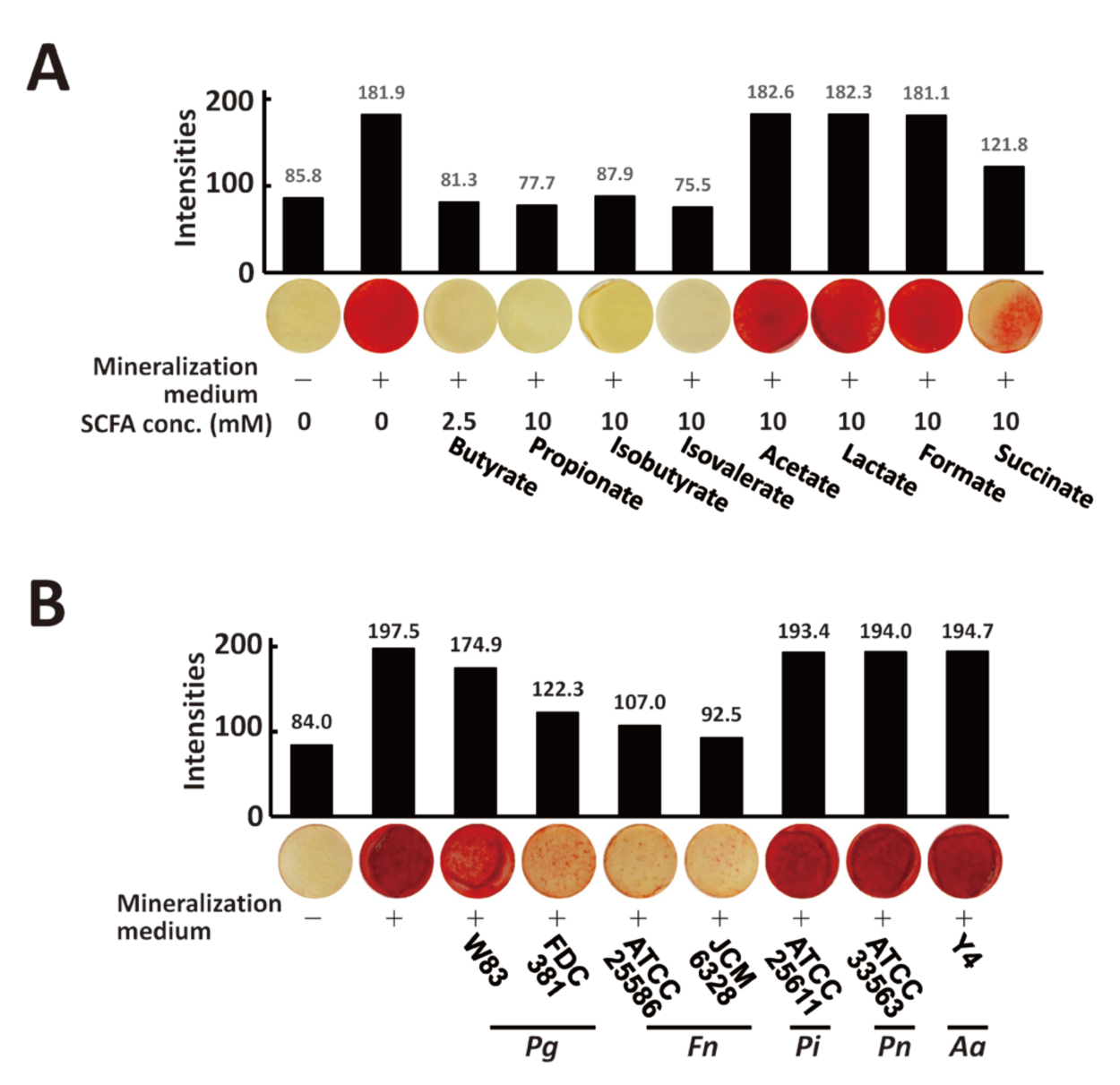
To investigate the effect of SCFA treatment on the osteogenic function of osteoblasts, murine osteoblast-like MC3T3-E1 clone NDC10E1cells were treated with each SCFA and mineralization abilities were evaluated using alizarin red S staining. While treatment of NDC10E1 cells with butyrate, propionate, isobutyrate, isovalerate, and succinate reduced mineralization induced by the mineralization medium (Fig. 3A), treatment with acetate, lactate, or formate did not alter the mineralization of NDC10E1 cells in the presence of a mineralization medium (Fig. 3A). Next, the effects of SCFA-mixtures that mimic oral-bacterial culture supernatants were examined. Mineralization levels of cells treated with SCFA mixtures mimicking P. gingivalis strains W83, FDC381, F. nucleatum strains ATCC25586, and JCM6328 in the presence of a mineralization medium were 11.4, 38.1, 45.8, and 53.2% lower, respectively, when compared to untreated cells (Fig. 3B). Treatment with SCFA mixture mimicking other bacterial culture supernatants (P. intermedia, P. negrescens, and A. actinomycetemcomitans) did not alter mineralization level (Fig. 3B).
Discussion
It was demonstrated that treatment of macrophage-like Raw264.7 cells with bacterial culture supernatants from P. gingivalis and F. nucleatum, which contain high concentrations of butyrate, propionate, isobutyrate, and isovalerate, suppressed LPS-induced iNOS production (Fig. 1A-C). These data suggested that bacteria-producing SCFAs, particularly butyrate, propionate, isobutyrate, and isobutyrate, have reducing effects on LPS-induced M1-like macrophage activation. Macrophages are functionally divided into two types. M1-type macrophages activated by LPS and interferon (IFN)-γ strongly produce inflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-1, and IL-6, resulting in the activation of inflammation, immune response, and subsequent pathogen killing [10,11]. The induction of iNOS is a marker for macrophages of the M1 type. [10,11]. M2-type macrophages downregulate the production of inflammatory cytokines and upregulate anti-inflammatory cytokines, resulting in inhibition of inflammation and subsequent tissue restoration [11]. Removal of the blood clot exposes the extracted cavities to oral bacteria, allowing for the easy flow of bacteria into the cavities. Since oral bacteria in the cavities metabolize residual food remnants and produces high concentrations of SCFAs, the data presented suggest that SCFAs produced by P. gingivalis and F. nucleatum may suppress the activation of M1-type macrophages, thereby reducing inflammation. SCFAs produced by oral bacteria such as butyrate, propionate, isobutyrate, and isovalerate may have strong effects to suppress the inflammation and subsequent immune response. Since Levitin et al.’s study suggests that there is a relationship between alveolar osteitis and immune-compromised patients [12], the immune response suppressed by bacteria-derived SCFAs may play an important role in the development of alveolar osteitis.
SCFAs produced by oral bacteria can affect the bone surface of sockets that do not have a blood clot. SCFA mixtures that mimic culture supernatants of P. gingivalis and F. nucleatum almost completely suppressed RANKL-induced osteoclast-like cell formation (Fig. 2B). These culture supernatants contain high or relatively high concentrations of butyrate, propionate, isobutyrate, and isovalerate, which almost completely suppressed RANKL-induced osteoclast formation (Table 1, Fig. 2A). Mimic of bacterial culture supernatants of P. gingivalis strain FDC381 and F. nucleatum strongly, but not completely, reduced mineralization (Fig. 3B). These data suggest that treatment of the bone surface with SCFAs in culture supernatants of P. gingivalis strain FDC381 and F. nucleatum may induce a gradual increase of bone mineralization. The culture supernatant mimic of P. gingivalis strain W83 almost completely reduced RANKL-induced osteoclastgenesis but resulted in only a small decrease in mineralization by osteoblasts. This SCFA mixture may exhibit much stronger bone mineralization compared to those of P. gingivalis strain FDC381 and F. nucleatum. By contrast, treatment with SCFA mixtures of P. intermedia, P. nigrescens, and A. actinomycetemcomitans significantly reduced the number of TRAP-positive multinuclear giant cells (but many TRAP-positive small cells remained) and maintained ordinary levels of mineralization by osteoblasts. Although this condition may induce bone mineral content, it depends on the bone resorptive abilities of the single nuclear cells. The effects of oral bacterial products on bone metabolism seem to be dependent on SCFA ingredients produced by oral bacteria.
It can be concluded that SCFAs produced by P. gingivalis and F. nucleatum can inhibit endotoxin-induced inflammation in the tooth sockets when the blood clot was removed from the cavities after extraction. Since obvious signs of infectious inflammation such as fever, leukocytosis, and lymphadenopathy are rare in alveolar osteitis patients [Rohe C et al., Alveolar Osteitis, StatPearls, 2023 Jan-], inhibitory effects of the SCFAs on LPS-induced M1 macrophage activation may correspond to the symptoms of alveolar osteitis. However, the name “alveolar osteitis” may not match with its pathological condition. P. gingivalis and F. nucleatum-producing SCFAs may add mineralized tissue on the alveolar bone walls of the socket. The increased cavity bone wall thickness due to SCFA effect may decrease blood supplies to the cavities, leading to a decrease in immune cell migration to the cavities. This condition may lead to the survival of bacteria in the cavity, causing poor oral hygiene and halitosis. Since these findings align with the physical observation of alveolar osteitis, SCFAs produced by oral bacteria in the tooth extraction cavities after the loss of blood clots might have the potential to contribute to the development of some of the symptoms of alveolar osteitis. Further studies are needed for understanding the relationship between SCFAs and alveolar osteitis.
As described in the previous paragraph, SCFAs produced by oral bacteria provide a place for bacteria to easily grow, resulting in possible poor oral hygiene with bad breath. Since several receptors that recognize short-chain fatty acids (FFA2, FFA3, and GPR109a) have been reported [13], inhibition of SCFA receptor signaling might somehow control the halitosis. However, since the signaling pathway involved in the SCFA-induced phenomena observed in the present study is unclear, further research into the signaling mechanisms is needed. The present study also did not investigate the role of SCFAs on severe pain associated with alveolar osteitis, which is the most unpleasant symptom of alveolar osteitis. Thus, research focusing on the role of SCFAs produced by oral bacteria in pain-generating mechanisms during alveolar osteitis may be the next challenge.
Abbreviations
FBS: fetal bovine serum; IFN: interferon; iNOS: inducible nitric oxide synthase; LPS: lipopolysaccharide; MEMα: minimum essential medium α; RANKL: receptor activator of nuclear factor-kappa B ligand; SCFA: short-chain fatty acid; TNF: tumor necrosing factor; TRAP: tartrate-resistant acid phosphatase.
Conflicts of Interest
All authors declare no potential conflict of interest with respect to the research, authorship, and publication of this article.
Funding
This work was supported by the KAKENHI (20K09913 and 23K09222), and the Uemura (H.T., 2021) and the Sato Funds (H.T., 2023), Nihon University School of Dentistry, and a grant from the Dental Research Center Nihon University School of Dentistry (H.T., 2022).
Author contributions
TA: investigation, data acquisition, and writing; AT: conceptualization, data acquisition, and methodology; YM: conceptualization and review; HY: data acquisition; MT: data acquisition; KM: review; KM: review; YY: review; HT*: conceptualization, methodology, writing, review, editing, and supervision; All authors read and approved the final version of the manuscript.
ORCID iD
1,2)TA: deta20001@g.nihon-u.ac.jp, NA
3)AT: deay17066@g.nihon-u.ac.jp, NA
4)YM: mikami-yoshikazu@med.niigata-u.ac.jp, NA
3)HY: http.xxx.hiro@gmail.com, NA
5,6)MT: tamura.muneaki@nihon-u.ac.jp, https://orcid.org/0000-0002-8637-1579
7)KM: matsumoto.kunihito@nihon-u.ac.jp, https://orcid.org/0000-0002-4045-976X
1,8)KM: deki20020@g.nihon-u.ac.jp, NA
2)YY: yonehara.yoshiyuki@g.nihon-u.ac.jp, https://orcid.org/0000-0002-3626-9023
9,10)HT*: tsuda.hiromasa@nihon-u.ac.jp, https://orcid.org/0000-0002-2047-2262
Acknowledgments
The authors would like to thank Editage (www.editage.com) for English language editing.
Data Availability Statements
Data generated during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article.
References
- 1. Chow O, Wang R, Ku D, Huang W (2020) Alveolar osteitis: a review of current concepts. J Oral Maxillofac Surg 78, 1288-1296.
- 2. Ghosh A, Aggarwal VR, Moore R (2022) Aetiology, prevention and management of alveolar osteitis-A scoping review. J Oral Rehabil 49, 103-113.
- 3. Blum IR (2002) Contemporary views on dry socket (alveolar osteitis): a clinical appraisal of standardization, aetiopathogenesis and management: a critical review. Int J Oral Maxillofac Surg 31, 309-317.
- 4. Nitzan DW (1983) On the genesis of “dry socket”. J Oral Maxillofac Surg 41, 706-710.
- 5. Amaratunga NA, Senaratne CM (1988) A clinical study of dry socket in Sri Lanka. Br J Oral Maxillofac Surg 26, 410-418.
- 6. Krakowiak PA (2011) Alveolar osteitis and osteomyelitis of the jaws. Oral Maxillofac Surg Clin North Am 23, 401-413.
- 7. Imai K, Ochiai K, Okamoto T (2009) Reactivation of latent HIV-1 infection by the periodontopathic bacterium Porphyromonas gingivalis involves histone modification. J Immunol 182, 3688-3695.
- 8. Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48, 452-458.
- 9. Pöllänen MT, Salonen JI, Uitto VJ (2003) Structure and function of the tooth-epithelial interface in health and disease. Periodontol 2000 31, 12-31.
- 10. Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11, 889-896.
- 11. Gordon S, Martinez FO (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32, 593-604.
- 12. Levitin SA, Jeong IC, Finkelstein J (2019) Mining electronic dental records to identify dry socket risk factors. Stud Health Technol Inform 262, 328-331.
- 13. Priyadarshini M, Kotlo KU, Dudeja PK, Layden BT (2018) Role of short-chain fatty acid receptors in intestinal physiology and pathophysiology. Compr Physiol 8, 1091-1115.
 https://orcid.org/0000-0002-8637-1579
https://orcid.org/0000-0002-8637-1579
 https://orcid.org/0000-0002-4045-976X
https://orcid.org/0000-0002-4045-976X
 https://orcid.org/0000-0002-3626-9023
https://orcid.org/0000-0002-3626-9023
 https://orcid.org/0000-0002-2047-2262
https://orcid.org/0000-0002-2047-2262




