2013 年 37 巻 4 号 p. D12-999
2013 年 37 巻 4 号 p. D12-999
The action mechanism of thiobencarb was studied by examining the inhibitory effects of this herbicide on the biosynthesis of very-long-chain fatty acids (VLCFAs). Thiobencarb treatment decreased VLCFAs, such as C20:0, C20:1, C22:0, C24:0, C24:1 and C26:0 fatty acids, and increased long-chain-fatty acids and medium-chain-fatty acids, such as C14:0, C15:0, C18:0 and C18:1 fatty acids, which are precursors of VLCFAs, in barnyard millet cultured cells. Thiobencarb sulfoxide and sulfone potently inhibited VLCFA elongase (VLCFAE) activity in the microsomal fraction of etiolated barnyard millet seedlings, although thiobencarb itself slightly inhibited it. These results suggested that thiobencarb is a VLCFAE-inhibiting herbicide whose active forms are its oxidized metabolites, such as sulfoxide and sulfone. Thiobencarb sulfoxide inhibited the VLCFAE activity of the microsomal fraction of etiolated barnyard millet seedlings in a time-independent manner. This time-independent inhibition proposed a reversible inhibition mechanism of the VLCFAE by thiobencarb sulfoxide, likely with isoxazoline-type herbicides, such as pyroxasulfone, which have been classified into group K3 of the Herbicide Resistance Action Committee (HRAC) in the U.S. It is assumed that the time-independent reversible inhibition of VLCFAE is applicable to other thiocarbamate herbicides presently classified into group N of the HRAC.
Thiobencarb, S-(4-chlorobenzyl)-N,N-diethylthiocarbamate, is a herbicide developed by Kumiai Chemical Industry for crops such as rice, wheat, corn and soybean (Fig. 1A).1) This herbicide at the application rate of 1.5–7.5 kg a.i./ha provides good efficacy on both grass and broad leaf weeds, especially needle spikerush and barnyard grass.2) Barnyard grass from pre-emergence up to the three-leaf stage is effectively controlled by this herbicide. This herbicide shows excellent selectivity for rice plants. Differences in the physiological activity between rice and barnyard grass for absorbing and metabolizing this herbicide have been reported.3–5)
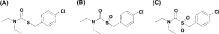
Thiobencarb does not inhibit the germination of seeds but potently inhibits the shoot elongation of germinated seeds. This herbicide belongs to the thiocarbamate herbicides and is classified into group N of the Herbicide Resistance Action Committee (HRAC) in the U.S.6) According to the HRAC, the target site of the group N herbicides is described as the lipid synthesis except for acetyl-CoA carboxylase (ACCase), indicating that their mode of action has not been completely elucidated.7,8) However, it has been reported that sulfoxide of pebulate, which is also a thiocarbamate herbicide, inhibits the incorporation of 14C-labeled acetic acid into very-long-chain fatty acids (VLCFAs) in some plants.9,10) Therefore, the thiocarbamate herbicides were presumed to inhibit the biosynthesis of VLCFAs in plants.9,10)
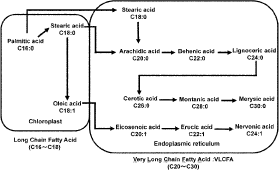
The biosynthesis of plant VLCFAs with chain lengths of 20 or more carbon atoms is catalyzed by VLCFA elongases (VLCFAEs) (Fig. 2).11–18) It has been revealed that there are lots of VLCFAEs in Arabidopsis and they catalyze multiple elongation steps in the biosynthesis of VLCFAs.11–18)
In this paper, we examined the effects of thiobencarb on the content of VLCFAs and subsequently, the inhibitory effects of thiobencarb and its oxidized metabolites on VLCFAE activity of plant to elucidate its mode of action.
Thiobencarb, thiobencarb sulfoxide and thiobencarb sulfone (Fig. 1) were synthesized at KI Chemical Research Institute Co., Ltd. (Shizuoka, Japan) and provided to our institute. Seeds of rice (Oryza sativa L. var. Nippon-Bare) and barnyard millet (Echinochloa frumentacea Link) were commercially obtained (Ohishi Nursery Company, Shizuoka, Japan). Seeds of late watergrass (Echinochloa oryzicola Vasing.) were picked from our seed farm and used in the experiments.
2. Effect of thiobencarb on the growth of plant shootsThe effects of thiobencarb on the shoot growth of rice, late watergrass and barnyard millet were examined by the method reported in a previous paper.19) Thiobencarb was dissolved in acetone and added to the medium. The final concentration of acetone was 0.1%. Shoot length was measured after 6 to 7 days of cultivation. Inhibition of the growth of plant shoots was calculated by comparing the shoot length of plants to that of the control grown in the absence of thiobencarb. The concentration required for 50% inhibition of the shoot growth (EC50) by thiobencarb was calculated by the probit method.
3. Effect of thiobencarb on the growth of barnyard millet cultured cellsBarnyard millet cultured cells were induced by the method reported by Xu et al.20) The effects of thiobencarb on the cell proliferation of cultured cells were examined according to the method for pyroxasulfone.19) Thiobencarb was dissolved in acetone and added to the medium. After 15 days of culture, the cultured cells, from which the liquid medium had been completely eliminated using a pipette, were transferred to a new container and weighed. Inhibition of cell proliferation by thiobencarb was calculated by comparing the cell growth to that of the control grown in the absence of thiobencarb.
4. Effects of thiobencarb on the biosynthesis of VLCFAs in barnyard millet cultured cellsQuantification of fatty acid contents in barnyard millet cultured cells was carried out with gas chromatography (GC) equipped with a flame ionization detector (FID) according to the method reported in the analysis of fatty acids with rice cultured cells.19,21) The significance of inter-group differences in fatty acid contents was analyzed by Williams’ test.
5. Preparations of microsomal fractions from etiolated barnyard millet seedlingsThirty grams of etiolated barnyard millet seedlings of approximately 10 cm in height, which were grown in the dark for 7 days at 27°C on vermiculite, was used in this experiment. Microsomal fractions (2 mL) of etiolated barnyard millet seedlings were prepared as reported previously.21) Microsomal suspensions were stored at −80°C until the enzyme assay.
6. Inhibition of microsomal VLCFAEs in etiolated barnyard millet seedlings by thiobencarb and its oxidized metabolitesVLCFAE activity was evaluated by examining the incorporation of [2-14C] malonyl-CoA into acyl-CoA. Behenoyl-CoA (C22:0-CoA) and lignoceroyl-CoA (C24:0-CoA) were purchased from Avanti POLAR LIPID (Alabaster, AL). The inhibitory effects of thiobencarb and its oxidized metabolites: sulfoxide and sulfone on VLCFAEs in etiolated barnyard millet seedlings were examined as reported previously.21) The zero-time reaction was used as the negative control. The inhibition of VLCFAE activities was determined by comparing the activities with thiobencarb and its oxidized metabolites to those of the positive control without chemicals. The concentrations required for 50% inhibition of VLCFAE activity (I50) were calculated by the probit method.
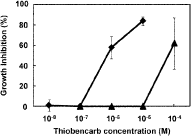
The shoot growth of late watergrass was inhibited by thiobencarb with the EC50 value of 9.2×10−7 M. Inhibition of shoot growth of rice was weaker than that of late watergrass, indicating the selectivity of thiobencarb between crop and weed (Fig. 3). The inhibition of shoot growth of barnyard millet (EC50, 1.2×10−6 M) was almost the same as that of late watergrass.

On the other hand, barnyard millet cultured cells showed only a slight sensitivity to thiobencarb, which was greatly distinct from its effect on the shoot growth (Fig. 4). This result indicated that the effects of thiobencarb on the biosynthesis of VLCFAs in the cultured cells could be examined with little effect of thiobencarb on cell proliferation even under high concentrations of thiobencarb that are lethal to shoot growth, as in the case of the VLCFAE-inhibiting herbicide, fenoxasulfone.22)
In barnyard millet cultured cells treated with thiobencarb, VLCFAs, such as C20:0, C20:1, C22:0, C24:0, C24:1 and C26:0 decreased, whereas long-chain-fatty acids and medium-chain-fatty acids, such as C14:0, C15:0, C18:0 and C18:1, which are precursors of VLCFAs, increased (Table 1). These results suggested that thiobencarb inhibits the biosynthesis of VLCFAs, which were incorporated into the plasma membrane of barnyard millet cultured cells, as indicated for the VLCFAE-inhibiting herbicide, fenoxasulfone.14–18,22) Considering the difference in the sensitivity of barnyard millet to thiobencarb between cultured cells and shoots as described above (Fig. 4), VLCFAs, which are significant components of the wax layer of plant cuticle, would be essential for the shoot elongation of barnyard millet but not for the proliferation of its cultured cells. Since thiocarbamate sulfoxides are presumed to be their herbicidally active metabolites,9,10) the effects of thiobencarb sulfoxide on barnyard millet cultured cells were also examined. As a result, the inhibition of proliferation of barnyard millet cultured cells by thiobencarb sulfoxide was almost the same as that by thiobencarb, and reduction of VLCFAs and accumulation of VLCFA precursors in barnyard millet cultured cells treated with thiobencarb sulfoxide were moderately lower than those in cells treated with thiobencarb (data not shown). It was considered that this difference was due to poorer uptake23) and/or more rapid metabolism of thiocarbamate sulfoxide than those of thiocarbamate itself.
| Fatty acid | Content (μg/g Fresh weight)a) | |||
|---|---|---|---|---|
| Thiobencarb | ||||
| 0 | 10−7 | 10−6 | 10−5 | |
| a) Data are expressed as the mean±SD of four independent experiments and asterisks indicate a significant difference from the respective control value by Williams’ test (* p<0.05, ** p<0.01). b) Not detected. | ||||
| C14:0 | 6.78±1.53 | 8.45±0.73 | 7.72±0.78 | 8.60±1.28* |
| C15:0 | 6.17±1.63 | 5.57±0.72 | 5.88±1.22 | 7.29±2.19 |
| C16:0 | 1040±83.7 | 988±24.1 | 928±104 | 1080±44.2 |
| C18:0 | 9.07±0.56 | 13.6±4.48 | 11.1±2.39 | 11.7±2.23 |
| C18:1 | 245±12.7 | 241±15.5 | 290±20.6** | 282±25.0** |
| C18:2 | 2500±241 | 2450±73.6 | 2360±209 | 2620±76.7 |
| C18:3 | 268±28.1 | 210±21.4 | 259±35.7 | 279±6.69 |
| C20:0 | 28.3±7.91 | 25.4±1.83 | 23.7±2.38 | 21.1±2.73** |
| C20:1 | 4.38±0.66 | 3.53±0.63 | 3.92±1.06 | 3.99±0.39 |
| C22:0 | 27.8±3.90 | 25.5±0.63 | 22.3±2.77** | 22.3±2.80** |
| C22:1 | n.d.b) | n.d. | n.d. | n.d. |
| C24:0 | 73.6±10.7 | 60.2±3.09* | 54.5±7.58** | 53.6±5.42** |
| C24:1 | 14.2±5.58 | 9.30±1.12 | 11.0±2.84 | 10.9±1.03 |
| C26:0 | 34.4±5.55 | 28.8±1.82* | 25.0±3.15** | 24.2±2.09** |
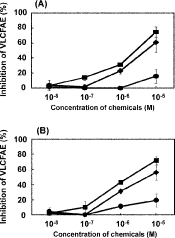
The microsomal VLCFAE activities in etiolated barnyard millet seedlings, which catalyze the elongation steps from C22:0 to C24:0 and C24:0 to C26:0, were slightly inhibited by thiobencarb, while its sulfoxide and sulfone potently inhibited those VLCFAE activities (Figs. 5A, 5B). The I50 values of the VLCFAE activity, which catalyzes the former elongation step, by thiobencarb sulfoxide and sulfone were 2.0×10−6 and 6.0×10−6 M, respectively, and those of the activity, which catalyzes the latter elongation step, by thiobencarb sulfoxide and sulfone were 2.3×10−6 and 5.3×10−6 M, respectively. These results not only suggested that thiobencarb is a VLCFAE-inhibiting herbicide but also indicated that its oxidized metabolites: sulfoxide and sulfone, are active compounds that have the ability to inhibit VLCFAE activity because thiocarbamate herbicide is known to be metabolized to its oxidized compounds in plants.24–27) However, considering the inhibitory potency of thiobencarb sulfoxide to VLCFAE activities (Figs. 5A, 5B), the reduction of VLCFAs and the accumulation of VLCFA precursors in barnyard millet cultured cells treated with thiobencarb seemed to be relatively low (Table 1). It is presumed that rapid degradation of thiobencarb to inactive compounds via thiobencarb sulfoxide weakened its effect on VLCFA biosynthesis in cultured cells.
Comparing the inhibition of VLCFAEs in barnyard millet by thiobencarb sulfoxide with that of shoot elongation of barnyard millet by thiobencarb, the former (I50) was approximately 2-fold weaker than the latter (EC50) (Figs. 3, 5A, 5B). Biosynthetic reactions of plant VLCFAs catalyzed by VLCFAEs proceeded sequentially (Fig. 2). The decrease of the acyl-CoA concentration caused by the inhibition of an elongation step affected the inhibition of the next elongation step, leading to stronger inhibition of the subsequent elongation step. Thus, each inhibitory effect on the VLCFAE activity, which catalyzes an elongation step in the biosynthesis of VLCFAs, synergistically affects the total inhibitory effects of thiobencarb on the biosynthesis of VLCFAs.22,28,29) Therefore, it is considered that the sequential blockade of VLCFA biosynthesis by thiobencarb sulfoxide, as indicated in the inhibition of two successive elongation steps (Figs. 5A, 5B), causes strong inhibition of shoot elongation by thiobencarb.
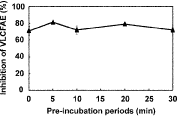
In general, VLCFAE-inhibiting herbicides contain a highly electrophilic carbon atom in their chemical structures. Accordingly, the VLCFAE inhibition mechanism has been assumed to be the nucleophilic reaction of the SH group of a cysteine residue in the active center of VLCFAEs with herbicides.28–30) In the case of such an inhibition mechanism, the VLCFAE activity would be inhibited in a time-dependent manner. Thiobencarb sulfoxide, however, inhibited the microsomal VLCFAE activity in barnyard millet, which catalyzes the elongation step from C24:0 to C26:0, in a time-independent manner (Fig. 6), as previously indicated for isoxazoline-type herbicides, such as pyroxasulfone and fenoxasulfone, which are classified into group K3 in the HRAC.21,22) This time-independent inhibition proposed a reversible inhibition mechanism of VLCFAE by thiobencarb sulfoxide. It is assumed that this time-independent reversible inhibition of VLCFAE is applicable to other thiocarbamate herbicides presently classified into group N of the HRAC. However, further studies will be necessary to determine whether this inhibition mechanism is applied to all of VLCFAE-inhibiting herbicides or not.
We express our thanks to Dr. Megumi Terada of our company for data analysis by Williams’ test.