Introduction
Japanese foxtail (Alopecurus japonicus), a monocot weed from the Poaceae family, is a major grass weed widely distributed in winter wheat fields in China, Japan, Turkey, and other Asian countries.1) It is a prolific seed producer and a strong competitor, and it can substantially reduce the crop yields of winter wheat. This weed, which is among the top 10 weeds in winter wheat fields in eastern China, is very troublesome and difficult to control.2,3)
Acetolactate synthase (ALS) inhibitors, including mesosulfuron-methyl and pyroxsulam, are mainly used to effectively control A. japonicus and other gramineous weeds in wheat fields. Mesosulfuron-methyl is the most commonly used herbicide. It was introduced in China in 2003. Due to its low use rates, high efficacy, broad spectrum of weed control and favorable environmental profiles, mesosulfuron-methyl eventually became a popular herbicide in eastern China. However, with its wide and repeated use, mesosulfuron-methyl-resistant A. japonicus populations have appeared over the last 2–3 years, and mesosulfuron-methyl could no longer control A. japonicus as effectively as it previously had in Jiangsu province and Anhui province.
Resistance to ALS inhibitors has evolved rapidly since ALS-inhibiting herbicide resistance was first reported in rigid ryegrass (Lolium rigidum) in Australia in 1982.4) To date, 129 weed species with evolved resistance to ALS inhibitors have been reported worldwide.4) Several cases of weed resistance to mesosulfuron-methyl have been specifically documented as well. These involve Lolium multiflorum in the southern United States,5) Apera spica-venti in Poland,6) and Alopecurus myosuroides in the United Kingdom.7) Studies on weed resistance to mesosulfuron-methyl in China have not been reported.
In many cases, target-site modification in the ALS gene in weeds had resulted in resistance to ALS inhibitors and had reduced the sensitivity of ALS to herbicides. Currently, eight conserved amino acid residues in the ALS gene are known to confer ALS-inhibitor resistance in field-selected weed populations, including alanine 122, proline 197, alanine 205, aspartate 376, arginine 377, tryptophan 574, serine 653, and glycine 654 by the Arabidopsis numbering system.4) These substitutions have various resistance responses to different kinds of ALS inhibitors. Pro197 substitution usually results in high sulfonylurea resistance, and many substitutions have been reported for this amino acid residue.8–23)
This study determined the resistance of A. japonicus populations to mesosulfuron-methyl. Its objectives were to (1) characterize the degree of resistance in resistant and susceptible populations, and (2) test whether the resistance mechanism is due to a modified ALS.
Materials and Methods
1. Plant materials
Seeds of a resistant A. japonicus population, JS-5, were collected from winter wheat fields where the mesosulfuron-methyl failed to control A. japonicus after it was continuously applied for several years in Jiangsu province. Seedlings of the population were treated with mesosulfuron-methyl at the commercial rate of 18 g a.i./ha. Survivors were grown to maturity to produce bulked seeds, and these seeds were used for all subsequent experiments. Another population, JS-7, was collected from a remote, hilly location without history of ALS-inhibiting herbicide application and used as the susceptible control.
2. Whole-plant pot experiments
One mesosulfuron-methyl-resistant A. japonicus population (JS-5) and one susceptible population (JS-7) were used for the experiments. Seeds of these populations were germinated in 12-cm-diameter pots containing moist loam soil. Pots were placed in a greenhouse (temperature between 16 and 25°C, light quantum of 434.3 µmol/m/s, and 75% relative humidity). After emergence, the seedlings were thinned to 10 evenly sized plants per pot prior to herbicide application, watered, and fertilized as required. Mesosulfuron-methyl for the JS-5 population was applied at 0, 4.5, 9, 18, 36, 72, and 144 g a.i./ha. As for the JS-7 population, the doses administered to A. japonicus were 0, 0.28, 0.56, 1.13, 2.25, 4.5, and 9 g a.i./ha. Herbicide treatments were applied to A. japonicus at the third to fourth leaf stages using a compressed air, moving-nozzle cabinet sprayer equipped with one Teejet 9503EVS flat fan nozzle and calibrated to deliver 450 L·ha−1 at 0.275 MPa. Twenty-one days after treatment, plants were harvested at the soil surface, and their fresh weight was determined. All treatments were replicated three times, and the experiment was conducted twice.
Combined data from the two experimental runs were subjected to ANOVA, and dose-response relationships were examined using the Probit model (Eq. (1)) of SPSS Version 17.0 (SPSS Inc.)
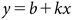 | (1) |
where y is the probit variable, b is the intercept, k is the regression coefficient, and x is the log 10 (dose). GR50 is the herbicide rate required for 50% growth reduction. The degree of resistance for the R population was determined by the resistance ratio (GR50 JS-5)/(GR50 JS-7).
3. ALS in vitro inhibition assays
Shoot materials from the JS-5 and JS-7 populations were harvested at the third to fourth leaf stages, frozen in liquid nitrogen immediately, and kept at −80°C. ALS extraction and in vitro herbicide inhibition assays were performed as described by Yu et al.24) ALS inhibition was established by a series of herbicide concentrations of mesosulfuron-methyl at 0, 0.001, 0.01, 0.1, 1, 10, and 100 µM. The experiment was conducted twice with three subsamples for each herbicide concentration. The I50 value (mesosulfuron-methyl concentration causing 50% inhibition of ALS activity) was estimated using the Probit model.
4. Genomic DNA extraction
Young shoot tissues measuring approximately 1 g from individual plants from the JS-5 and JS-7 populations were harvested and stored at −80°C. DNA was extracted from 100 mg of young shoot tissue from each plant using the CTAB method.25)
5. Primer design for ALS gene sequencing
One pair of primers (shown in Table 1) was designed using Primer Premier 5.0 to amplify the highly conserved region of the ALS encompassing the eight point mutations based on plant ALS sequences available in GenBank. The plant species used was A. myosuroides (AJ437300).
Table 1. Primers used to amplify the
A. japonicus ALS gene
| Primers |
Sequence (5′-3′) |
Amplicon size (bp) |
| Forward |
ATCAGGTGCTCAGCGGTGTC |
— |
| Reverse |
CCTTTAGGTCTTCTAGGTCG |
1843 bp |
The DNA engine Bio-RAD was used to amplify ALS gene fragments from A. japonicus genomic DNA. Polymerase chain reaction (PCR) was performed in a final volume of 25 µL, including 1 µL of genomic DNA (25 ng), 1 µL of each primer (20 µM), 2.5 µL of 10X Taq buffer (Mg2+ Plus, Tiangen Biotech, China), 2 µL of dNTP mixture (2.5 mM, Tiangen Biotech), and 0.5 µL of Taq Plus DNA Polymerase (2.5 U/µL, Tiangen Biotech). PCR was subjected to denaturation at 94°C for 5 min; 35 cycles at 94°C for 1 min, 56°C for 50 sec, and 72°C for 2 min; and a final step at 72°C for 10 min.
PCR products were detected using 1.5% agarose gel, and extracted using the TIANgel Midi Purification Kit (Tiangen Biotech). The desired PCR bands were cloned with the pEASY-T1 cloning vector (TransGen BioTech, China), after which the recombinant plasmids were introduced into competent Escherichia coli (Trans1-T1 Phage Resistant Chemically Competent Cell, Trans BioTech, China) according to the manufacturer’s instructions. Positive clones were sequenced on an ABI-PRISM 3730 DNA sequencer by Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. (China). Three biological replicates of each population were used for ALS gene amplification. At least five clones for each biological replicate were sent for sequencing and used to construct the ALS consensus sequence. Sequence data were aligned and compared using DNAMAN Version 5.2.2.
Results
1. Level of resistance to mesosulfuron-methyl
The ANOVA of pot experiments did not reveal any significant difference between the two experiments, and treatment means were averaged for both. The JS-5 population exhibited a high level of resistance to mesosulfuron-methyl. The GR50 values of the JS-5 and JS-7 populations were 99.86 and 0.59 g a.i./ha, respectively, and the resistance index was 169.25 compared with GR50 (JS-5)/GR50 (JS-7) (Table 2).
Table 2. Whole-plant pot experiments. GR
50 values for whole-plant response of standard susceptible and mesosulfuron-methyl-resistant (JS-5)
A. japonicus populations
| Population |
Regression equation |
R
2
|
GR50a (g a.i. ha−1) |
LLb |
ULb |
R/Sc |
| JS-7(S) |
y=0.338+1.465x |
0.97 |
0.59 |
0.46 |
0.72 |
— |
| JS-5(R) |
y=−3.883+1.942x |
0.98 |
99.86 |
75.31 |
130.35 |
169.25 |
a GR50 is the ALS inhibiting herbicide rate required for 50% growth reduction.b 95% confidence interval: LL=lover limit; UL=upper limit.c R/S ratios were calculated on the basis of GR50 values of accession JS-5 relative to the susceptible control population JS-7.
To determine whether the resistance is due to an insensitive ALS target enzyme, the ALS activities of the JS-5 and JS-7 populations were assayed in the presence of mesosulfuron-methyl in increasing doses (Fig. 1). ALS activity in the JS-5 population was less affected by increased mesosulfuron-methyl concentration than that in the JS-7 population. In addition, the concentration of mesosulfuron-methyl required to inhibit 50% (I50) enzyme activity in the JS-5 population was 0.628 µM, whereas that in the JS-7 population was 0.041 µM, which means that the rate causing 50% inhibition of ALS activity (I50) for the resistant (JS-5) population was 15 times higher than that for the susceptible (JS-7) population. The results with extracted enzymes were in accordance with the whole-plant results (Table 2), suggesting that target-site-based resistance was involved.

Fig. 1. In vitro ALS enzyme activities of the JS-7 and JS-5 populations in the presence of increasing concentrations of mesosulfuron-methyl. ALS activity is expressed as the percentage of activity in the absence of herbicide (control activity). Each data point represents the mean±SE, and each experiment had three replicates.
To identify the molecular basis for the resistance, the ALS genes from JS-5 and JS-7 were cloned, sequenced and compared. DNA was extracted from the leaf tissue of individual plants from these populations. The total length of the conserved ALS gene was 1843 bp, encompassing the eight known point mutations for ALS-inhibiting herbicide resistance.
Based on comparisons of the ALS gene sequences of the susceptible and resistant populations with Arabidopsis, Pro at position 197 in Domain A was replaced by threonine (Thr) in the resistant population JS-5. To exclude the possibility that this was an accidental mutation at position 197, we amplified the fragment containing Domain A from 30 random seedlings of each population. Comparisons of these sequences showed Pro at position 197 in the JS-7 population, but Thr in the JS-5 population (Fig. 2).

Fig. 2. DNA and derived amino acid sequences of the ALS genes from the susceptible and resistant populations of A. japonicus. The amino acid shown in bold encode Pro197 in the susceptible population and the mutations in the resistant population with Arabidopsis.
Discussion
The whole-plant pot experiments showed that the JS-5 population was highly resistant to mesosulfuron-methyl as compared with the susceptible population JS-7 (>100-fold). Lamego et al. reported that the appearance of the highly resistant weed populations was attributed to lack of herbicide rotation and strong selection pressure.26) Rubin suggested that resistant weed populations might appear after more than three years of continuous application of ALS-inhibiting herbicides.27) Mesosulfuron-methyl was primarily applied in wheat fields to selectively control grass weed in 2003 in Jiangsu province. Since 2010, farmers have observed that mesosulfuron-methyl at recommended rates fails to control A. japonicus in wheat fields after several years of successful control.
The ALS in vitro inhibition assays showed that the ALS sensitivity to mesosulfuron-methyl was significantly reduced in the JS-5 population as compared with the JS-7 population. In many cases, evolved resistance is due to a reduction in target-enzyme sensitivity conferred by distinct mutations within the ALS gene.24) A Pro197 to Thr mutation was found in the JS-5 population as compared with the JS-7 population. This mutation confers resistance to sulfonylurea herbicides in several grass weeds such as barley grass (Hordeum leporinum), wind bentgrass (A. spica-venti) and blackgrass (A. myosuroides).16–18) Therefore, the Pro197 to Thr mutation is likely the molecular mechanism of mesosulfuron-methyl resistance in A. japonicus.
To date, 24 resistance-endowing gene mutations at eight conserved amino acid residues (122, 205, 197, 376, 377, 574, 653, and 654) in the ALS gene have been identified in resistant weeds.28) Twelve different mutations at ALS position 197 have been found among several key broadleaved and grass weed species.29) At Pro197, substitution with Thr is a common mutation. Like the Pro197 Ser mutation, the Pro197 to Thr mutation requires only one nucleotide mutation, whereas some of the Pro197 amino acid substitutions (e.g., Ile, Lys, Met, and Trp) require two nucleotide changes.30) This may be one of the reasons why the Pro197 Thr mutation was more frequently observed in ALS-inhibitor-resistant weeds.
Different mutations in ALS genes may confer different resistance and cross-resistance patterns to ALS-inhibitors. Generally, Pro197-Thr mutation confers SU and TP resistance. In addition, Hordeum leporinum and Apera spica-venti were found to be resistant to IMI and SCT, respectively.17,18) Our experiment showed that the JS-5 population was also resistant to chlorsulfuron and metsulfuron-methyl (data not shown). Now, the alternative use of herbicides of different action modes should be taken into account to avoid further expansion and spread of mesosulfuron-methyl-resistant populations. Meanwhile, a single herbicide cannot be used frequently over the long term or multiple herbicide resistance will evolve.
This study characterized, for the first time, the molecular basis of mesosulfuron-methyl resistance in A. japonicus. In summary, the results indicated that mesosulfuron-methyl resistance in A. japonicus is based on an altered ALS gene conferred by a single-point mutation at position 197.
Acknowledgment
The authors gratefully acknowledge all the workers for assistance in conducting this research. This research was funded by National Natural Science Foundation of China (No. 31171866 and No. 31201529) and Special Fund for Agroscientific Researchin the Public Interest (No. 201303031).
References
- 1) C. Yang, L. Dong, J. Li and S. R. Moss: Weed Sci. 55, 537–540 (2007).
- 2) I. A. Mohamed, R. Li, Z. You and Z. Li: Weed Sci. 60, 167–171 (2012).
- 3) H. Tang, J. Li, L. Dong, A. Dong, B. Lü and X. Zhu: Pest Manag. Sci. 68, 1241–1247 (2012).
- 4) I. M. Heap: International Survey of Herbicide Resistant Weeds. www.weedscience.org. Accessed: February 1 (2013).
- 5) Y. I. Kuk and N. R. Bugos: Pest Manag. Sci. 63, 349–357 (2007).
- 6) M. Krysiak, S. W. Gawronski, K. Adamczewski and R. Kierzek: J. Plant Prot. Res. 51, 261–267 (2011).
- 7) R. Marshall and S. R. Moss: Weed Res. 48, 439–447 (2008).
- 8) T. Jin, J. Liu, Z. Huan, C. Wu, Y. Bi and J. Wang: Pestic. Biochem. Physiol. 100, 160–164 (2011).
- 9) Q. Yu, I. Abdallah, H. Han, M. Owen and S. B. Powles: Planta 230, 713–723 (2009).
- 10) H. Cui, C. Zhang, H. Zhang, X. Liu, Y. Liu, G. Wang, H. Huang and S. Wei: Weed Sci. 56, 775–779 (2008).
- 11) N. S. Kaloumenos, C. A. Dordas, G. C. Diamantidis and I. G. Eleftherohorinos: Weed Sci. 57, 362–368 (2009).
- 12) L. Scarabel, A. Locascio, A. Furini, M. Sattin and S. Varotto: Pest Manag. Sci. 66, 337–344 (2010).
- 13) S. I. Warwick, C. A. Sauder and H. J. Beckie: Weed Sci. 58, 244–251 (2010).
- 14) R. Marshall, R. Hull and S. R. Moss: Weed Res. 50, 621–630 (2010).
- 15) Q. Yu, H. Han and S. B. Powles: Pest Manag. Sci. 64, 1229–1236 (2008).
- 16) D. Massa, B. Krenz and R. Gerhards: Weed Res. 51, 294–303 (2011).
- 17) C. Délye and K. Boucansaud: Weed Res. 48, 97–101 (2008).
- 18) M. J. Owen, D. E. Goggin and S. B. Powles: Pest Manag. Sci. 68, 757–763 (2012).
- 19) H. Cui, X. Li, G. Wang, J. Wang, S. Wei and H. Cao: Pestic. Biochem. Physiol. 102, 229–232 (2012).
- 20) K. W. Park, J. M. Kolkman and C. A. Mallory-Smith: Can. J. Plant Sci. 92, 303–309 (2012).
- 21) D. Zheng, G. R. Kruger, S. Singh, V. M. Davis, P. J. Tranel, S. C. Weller and W. G. Johnson: Pest Manag. Sci. 67, 1486–1492 (2011).
- 22) S. Intanon, A. Perez-Jones, A. G. Hulting and C. A. Mallory-Smith: Weed Sci. 59, 431–437 (2011).
- 23) P. Boutsalis, J. Karotam and S. B. Powles: Pestic. Sci. 55, 507–516 (1999).
- 24) Q. Yu, J. K. Nelson, M. Q. Zheng, M. Jackson and S. B. Powles: Pest Manag. Sci. 63, 918–927 (2007).
- 25) J. J. Doyle and J. L. Doyle: Focus 12, 13–15 (1990).
- 26) F. P. Lamego, D. Charlson, C. A. Delatorre, N. R. Burgos and R. A. Vidal: Weed Sci. 57, 474–481 (2009).
- 27) B. Rubin: Z. Pflanzenkr. pflanzenschutz 15, 17–32 (1996).
- 28) Q. Yu, H. Han, M. Li, E. Purba, M. J. Walsh and S. B. Powles: Weed Res. 52, 178–186 (2012).
- 29) S. S. Kaundun, R. P. Dale and G. C. Bailly: Weed Sci. 60, 172–178 (2012).
- 30) S. B. Powles and Q. Yu: Annu. Rev. Plant Biol. 61, 317–347 (2010).