2013 年 38 巻 2 号 p. 78-84
2013 年 38 巻 2 号 p. 78-84
As an important class of pesticides, organophosphorus compounds display a wide range of biological activities and have attracted considerable attention as the main source of lead compounds in agrochemicals. A detailed study of acylphosphinates and acylphosphonates revealed that these analogues of pyruvate could be designed as mechanism-based inhibitors of pyruvate dehydrogenase complex (PDHc). However, they are not active enough for full development as herbicides.1–3) These findings prompted us to perform our own study of novel PDHc inhibitors with potential as herbicides. In our previous work, a series of alkylphosphonic acid derivatives with general structure I (Fig. 1) was designed and synthesized.4–8) The bioassay results showed that some of them exhibited higher herbicidal activities. Among these alkylphosphonates, compound I-1 (Clacyfos), a competitive inhibitor of PDHc, was found to be the most effective compound against broadleaf weeds; it has been given temporary registration from the Institute for the Control of Agrochemicals, Ministry of Agriculture (ICAMA) in China.8)

On a different front, bioisosterism is an important concept of bioactive compound design.9,10) The phosphinate group is often used for bioisosteric replacement of the phosphonate group in bioactive molecules to obtain more bioactivity or safety, which encouraged us to replace the phosphonate group with the phosphinate group in structure I and further study the relationship of structure-herbicidal activity. Herein, we report the synthesis of the new O-methyl methyl[1-(substituted phenoxyacetoxy)alkyl]phosphinates II (Fig. 1) and evaluate its herbicidal activity.
1H and 31P NMR spectra were recorded on a Varian Mercury-Plus 200 spectrometer with CDCl3 as the solvent and TMS as the internal standard. Mass spectra were measured on a Finnigan TraceMS 2000 spectrometer. Infrared spectra were recorded in potassium bromide disks on a Nicolet Avatar 360 FTIR spectrometer. Elemental analysis was performed with an Elementar Vario EL III elementary analyzer. Melting points (mp) were measured on an electrothermal melting point apparatus and the temperatures were not corrected.
All of the solvents were anhydrous. Triethylamine and thionyl chloride were distilled before the reaction.
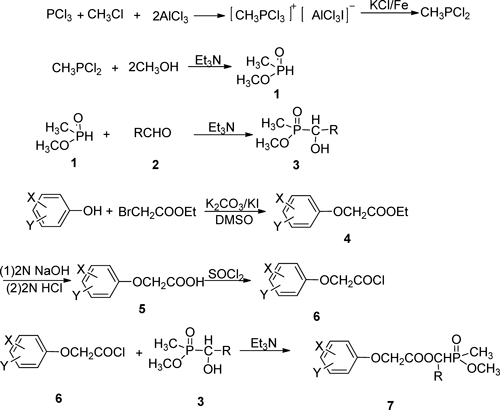
2. Chemical synthesis 2.1. Synthesis of O-methyl 1-hydroxyalkylphosphinates (3)O-methyl 1-hydroxyalkylphosphinates 3 could be prepared by the reaction of O-methyl methylphosphinate and several kinds of aldehydes using triethylamine as catalyst with a yield of 70–90% racemic mixture, according to the literature.13–15)
2.2. Synthesis of compounds 5 and 6The substituted phenoxyacetic acid 5 was synthesized by a standard method.5) The corresponding substituted phenoxyacetyl chloride 6 could be easily obtained as a yellow liquid with a 90% yield by treating compound 5 with thionyl chloride.
2.3. General procedure for synthesis of compound 7To a stirred mixture of O-methyl 1-hydroxyalkylphosphinates 3 (0.005 mol) and triethylamine (0.008 mol) in anhydrous dichloromethane (15 mL), a solution of substituted phenoxyacetyl chloride 6 (0.006 mol) in dichloromethane (15 mL) was added dropwise at a temperature below 5°C. The resulting mixture was stirred at ambient temperature for 3–5 hr; then washed separately with 0.1 mol/L HCl, saturated NaHCO3, and brine; dried; and evaporated. The residue was chromatographed on silica gel using acetone–petroleum ether (2 : 3) as the eluent to afford the compounds 7a–z as racemic mixtures.
O-methyl [(2,4-dichlorophenoxyacetoxy)(phenyl)methyl](methyl)phosphinate (7a) Colourless oil: 1H NMR (CDCl3, 200 MHz): δ=1.38 (d, 3H, –CH3, J=16 Hz), 3.64 (d, 3H, –OCH3, J=12 Hz), 4.86 (s, 2H, CH2), 6.29 (dd, 1H, CH, J=12, 14 Hz), 6.82–7.43 (m, 8H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=46.92; MS m/z (%): 402 (M+ 14.35), 175 (76.64), 93 (60.25), 77 (81.02), 43 (100.00); IR (ν, cm−1): 3062 (Ph-H), 1756 (C=O), 1295, 894 (P–C), 1220, 1070 (C–O–C), 1174 (P=O), 1102 (C–O), 1029 (P–O–C); Calcd. for C17H17Cl2O5P: C 50.64, H 4.25; Found: C 50.94, H 4.64.
O-methyl [(2,4-dichlorophenoxyacetoxy)(4-methylphenyl)methyl](methyl)phosphinate (7b) Colourless oil: 1H NMR (CDCl3, 200 MHz): δ=1.38 (d, 3H, –CH3, J=14 Hz), 2.35 (s, 3H, –C6H4CH3), 3.64 (d, 3H, –OCH3, J=10 Hz), 4.83 (s, 2H, CH2), 6.17 (dd, 1H, CH, J=11, 13 Hz), 6.73–7.42 (m, 7H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=47.21; MS m/z (%): 416 (M+ 4.12), 175 (15.28), 93 (17.93), 77 (16.78), 47 (100.00); IR (ν, cm−1): 3032 (Ph-H), 1768 (C=O), 1300, 894 (P–C), 1236, 1083 (C–O–C), 1175 (P=O), 1104 (C–O), 1040 (P–O–C); Calcd. for C18H19Cl2O5P: C 51.82, H 4.59; Found: C 51.52, H 4.74.
O-methyl [(2-chlorophenyl)(2,4-dichlorophenoxyacetoxy)methyl](methyl)phosphinate (7c) Colourless oil: 1H NMR (CDCl3, 200 MHz): δ=1.47 (d, 3H, –CH3, J=15 Hz), 3.69 (dd, 3H, –OCH3, J=13, 15 Hz), 4.84 (s, 2H, CH2), 6.66 (dd, 1H, CH, J=12, 14 Hz), 6.73–7.46 (m, 7H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=46.10; MS m/z (%): 436 (M+ 8.85), 401 (100.00), 175 (89.50), 93 (93.05), 77 (72.75); IR (ν, cm−1): 3058 (Ph-H), 1754 (C=O), 1300 (P–C), 1233, 1079 (C–O–C), 1180 (P=O), 1104 (C–O), 1035 (P–O–C); Calcd. for C17H16Cl3O5P: C 46.65, H 3.7; Found: C 46.42, H 3.7.
O-methyl [(4-chlorophenyl)(2,4-dichlorophenoxyacetoxy)methyl](methyl)phosphinate (7d) Colourless oil: 1H NMR (CDCl3, 200 MHz): δ=1.36 (d, 3H, –CH3, J=15 Hz), 3.61 (d, 3H, –OCH3, J=13 Hz), 4.80 (s, 2H, CH2), 6.12 (dd, 1H, CH, J=12, 15 Hz), 6.67–7.38 (m, 7H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=46.90; MS m/z (%): 436 (M+ 4.20), 233 (11.56), 175 (22.10), 93 (84.48), 77 (100.00); IR (ν, cm−1): 3072 (Ph-H), 1770 (C=O), 1301, 893 (P–C), 1237, 1087 (C–O–C), 1172 (P=O), 1104 (C–O), 1040 (P–O–C); Calcd. for C17H16Cl3O5P: C 46.65, H 3.7; Found: C 46.47, H 3.7.
O-methyl [(2,4-dichlorophenoxyacetoxy)(4-methoxyphenyl)methyl](methyl)phosphinate (7e) Colourless oil: 1H NMR (CDCl3, 200 MHz): δ=1.39 (d, 3H, –CH3, J=16 Hz), 3.62 (dd, 3H, –OCH3, J=4, 11 Hz), 3.79 (s, 3H, –OCH3), 4.80 (s, 2H, CH2), 6.09 (dd, 1H, CH, J=12, 15 Hz), 6.66–7.38 (m, 7H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=47.00; MS m/z (%): 432 (M+ 3.94), 175 (31.64), 93 (51.25), 77 (100.00), 63 (54.50); IR (ν, cm−1): 3074 (Ph-H), 1765 (C=O), 1301, 892 (P–C), 1251, 1082 (C–O–C), 1173 (P=O), 1105 (C–O), 1036 (P–O–C); Calcd. for C18H19Cl2O6P: C 49.89, H 4.42; Found: C 49.84, H 4.42.
O-methyl [(2,4-dichlorophenoxyacetoxy)(3-nitrophenyl)methyl](methyl)phosphinate (7f) Yellow oil: 1H NMR (CDCl3, 200 MHz): δ=1.47 (d, 3H, –CH3, J=15 Hz), 3.68 (dd, 3H, –OCH3, J=13, 15 Hz), 4.97 (s, 2H, CH2), 6.34 (dd, 1H, CH, J=13, 15 Hz), 6.82–8.28 (m, 7H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=44.40; MS m/z (%): 447 (M+23.03), 229 (65.36), 175 (49.97), 93 (100.00), 77 (50.20); IR (ν, cm−1): 3061 (Ph-H), 1758 (C=O), 1300, 889 (P–C), 1240, 1078 (C–O–C), 1170 (P=O), 1102 (C–O), 1038 (P–O–C), 1542, 1350 (NO2); Calcd. for C17H16Cl2NO2P: C 45.56, H 3.60; Found: C 45.761, H 3.18.
O-methyl [1-(2,4-dichlorophenoxyacetoxy)butyl](methyl)phosphinate (7g) White solid: 1H NMR (CDCl3, 200 MHz): δ=0.93 (t, 3H, –CH3, J=7 Hz), 1.38 (d, 3H, –CH3, J=14 Hz), 1.46–1.79 (m, 4H, CH2), 3.72 (d, 3H, –OCH3, J=11 Hz), 4.79 (s, 2H, CH2), 5.30–5.48 (m, 1H, CH), 6.75–7.41 (m, 3H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=48.75, 50.42; MS m/z (%): 368 (M+ 17.40), 175 (36.28), 121 (100.00), 93 (96.35), 63 (39.00); IR (ν, cm−1): 3061 (Ph-H), 1764 (C=O), 1307, 876 (P–C), 1214, 1087 (C–O–C), 1180 (P=O), 1108 (C–O), 1034 (P–O–C); Calcd. for C14H19Cl2O5P: C 45.55, H 5.19; Found: C 45.40, H 5.10.
O-methyl [1-(2,4-dichlorophenoxyacetoxy)propyl](methyl)phosphinate (7h) Colourless liquid: 1H NMR (CDCl3, 200 MHz): δ=0.93 (t, 3H, –CH3, J=11 Hz), 1.45 (d, 3H, –CH3, J=15 Hz), 1.70–2.01 (m, 2H, CH2), 3.73 (dd, 3H, –OCH3, J=13, 15 Hz), 4.80 (s, 2H, CH2), 5.30–5.48 (m, 1H, CH), 6.76–7.42 (m, 3H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=49.05, 50.88; MS m/z (%): 354 (M+ 2.86), 175 (10.94), 93 (100.00), 77 (52.24), 63 (79.98); IR (ν, cm−1): 3077 (Ph-H), 1766 (C=O), 1302, 870 (P–C), 1212, 1085 (C–O–C), 1188 (P=O), 1105 (C–O), 1040 (P–O–C); Calcd. for C13H17Cl2O5P: C 43.97, H 4.83; Found: C 43.20, H 4.80.
O-methyl [1-(2,4-dichlorophenoxyacetoxy)ethyl](methyl)phosphinate (7i) Yellow oil: 1H NMR (CDCl3, 200 MHz): δ=1.46 (d, 3H, –CH3, J=16 Hz), 1.51–1.57 (m, 3H, –CH3), 3.74 (dd, 3H, –OCH3, J=13, 15 Hz), 4.77 (s, 2H, CH2), 5.29–5.43 (m, 1H, CH), 6.76–7.41 (m, 3H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=50.38, 51.46; MS m/z (%): 340 (M+ 2.24), 122 (50.59), 93 (100.00), 63 (82.86), 47 (56.82); IR (ν, cm−1): 3078 (Ph-H), 1765 (C=O), 1302, 882 (P–C), 1217, 1083 (C–O–C), 1189 (P=O), 1105 (C–O), 1036 (P–O–C); Calcd. for C12H15Cl2O5P: C 42.26, H 4.43; Found: C 41.95, H 4.71.
O-methyl methyl[(2-nitrophenoxyacetoxy)(phenyl)methyl]phosphinate (7j) Brown oil: 1H NMR (CDCl3, 200 MHz): δ=1.35 (d, 3H, –CH3, J=16 Hz), 3.61 (d, 3H, –OCH3, J=13 Hz), 4.89 (s, 2H, CH2), 6.18 (dd, 1H, CH, J=12, 14 Hz), 6.86–7.87 (m, 9H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=47.20; MS m/z (%): 379 (M+ 0.32), 152 (28.24), 122 (77.50), 93 (100.00), 77 (61.57); IR (ν, cm−1): 3066 (Ph-H), 1765 (C=O), 1525, 1353 (NO2), 1301, 892 (P–C), 1236, 1096 (C–O–C), 1168 (P=O), 1040 (P–O–C); Calcd. for C17H18NO7P: C 53.83, H 4.47, N 3.69; Found: C 52.57, H 4.89, N 3.80.
O-methyl methyl[(4-methylphenyl)(2-nitrophenoxyacetoxy)methyl]phosphinate (7k) Brown oil: 1H NMR (CDCl3, 200 MHz): δ=1.37 (d, 3H, –CH3, J=14 Hz), 2.33 (s, 3H, CH3), 3.64 (d, 3H, –OCH3, J=11 Hz), 4.92 (s, 2H, CH2), 6.14 (t, 1H, CH, J=13 Hz), 6.97–7.88 (m, 8H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=47.75, 48.05; IR (ν, cm−1): 3055 (Ph-H), 1752 (C=O), 1522, 1348 (NO2), 1298, 888 (P–C), 1221, 1092 (C–O–C), 1165 (P=O), 1033 (P–O–C); Calcd. for C18H20NO7P: C 54.96, H 5.13, N 3.56; Found: C 54.92, H 5.28, N 3.51.
O-methyl [(2-chlorophenyl)(2-nitrophenoxyacetoxy)methyl](methyl)phosphinate (7l) Brown oil: 1H NMR (CDCl3, 200 MHz): δ=1.43 (d, 3H, –CH3, J=14 Hz), 3.66 (dd, 3H, –OCH3, J=13, 15 Hz), 4.88 (s, 2H, CH2), 6.64 (dd, 1H, CH, J=12, 14 Hz), 6.91–7.88 (m, 8H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=46.95; MS m/z (%): 413 (M+ 0), 378 (100.00), 122 (46.78), 93 (72.54), 77 (38.20); IR (ν, cm−1): 3074 (Ph-H), 1768 (C=O), 1301, 891 (P–C), 1236, 1096 (C–O–C), 1167 (P=O), 1130 (C–O), 1038 (P–O–C), 1524, 1352 (NO2); Calcd. for C17H17ClNO7P: C 49.34, H 4.14, N 3.38; Found: C 49.27, H 4.16, N 3.51.
O-methyl [(4-chlorophenyl)(2-nitrophenoxyacetoxy)methyl](methyl)phosphinate (7m) Yellow oil: 1H NMR (CDCl3, 200 MHz): δ=1.38 (dd, 3H, –CH3, J=4, 15 Hz), 3.63 (dd, 3H, –OCH3, J=12, 14 Hz), 4.95 (s, 2H, CH2), 6.34 (dd, 1H, CH, J=12, 14 Hz), 6.99–7.87 (m, 8H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=47.47, 47.83; IR (ν, cm−1): 3060 (Ph-H), 1764 (C=O), 1298, 892 (P–C), 1229, 1090 (C–O–C), 1166 (P=O), 1037 (P–O–C), 1522, 1349 (NO2); Calcd. for C17H17ClNO7P: C 49.34, H 4.14, N 3.38; Found: C 49.27, H 4.15, N 3.47.
O-methyl methyl[(2-nitrophenoxyacetoxy)(4-methoxyphenyl)methyl]phosphinate (7n) Yellow oil: 1H NMR (CDCl3, 200 MHz): δ=1.37 (d, 3H, –CH3, J=14 Hz), 3.63 (d, 3H, –OCH3, J=16 Hz), 3.79 (s, 3H, –OCH3), 4.91 (s, 2H, CH2), 6.15 (t, 1H, CH, J=13 Hz), 6.87–7.88 (m, 8H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=47.47, 47.83; IR (ν, cm−1): 3061 (Ph-H), 1760 (C=O), 1299, 893 (P–C), 1249, 1094 (C–O–C), 1168 (P=O), 1038 (P–O–C), 1521, 1343 (NO2); Calcd. for C18H20NO8P: C 52.81, H 4.92, N 3.42; Found: C 52.82, H 5.07, N 3.58.
O-methyl methyl[(2-nitrophenoxyacetoxy)(3-nitrophenyl)methyl]phosphinate (7o) Brown oil: 1H NMR (CDCl3, 200 MHz): δ=1.83 (dd, 3H, –CH3, J=4, 15 Hz), 3.64 (dd, 3H, –OCH3, J=12, 14 Hz), 4.96 (s, 2H, CH2), 6.26 (t, 1H, CH, J=12 Hz), 6.95–8.17 (m, 8H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=45.89; IR (ν, cm−1): 3058 (Ph-H), 1762 (C=O), 1305, 889 (P–C), 1248, 1098 (C–O–C), 1169 (P=O), 1031 (P–O–C), 1528, 1341 (NO2); Calcd. for C17H17N2O9P: C 48.12, H 4.04, N 6.60; Found: C 47.90, H 4.13, N 6.65.
O-methyl methyl[1-(2-nitrophenoxyacetoxy)butyl]phosphinate (7p) Brown oil: 1H NMR (CDCl3, 200 MHz): δ=0.90 (t, 3H, –CH3, J=16 Hz), 1.41 (dd, 3H, –CH3, J=4, 15 Hz), 1.62, 1.97 (m, 4H, CH2), 3.67 (dd, 3H, –OCH3, J=12, 14 Hz), 4.81 (s, 2H, CH2), 5.16–5.44 (m, 1H, CH), 6.94–7.86 (m, 4H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=48.76, 50.42; IR (ν, cm−1): 3084 (Ph-H), 1764 (C=O), 1303, 875 (P–C), 1227, 1096 (C–O–C), 1188 (P=O), 1038 (P–O–C), 1525, 1353 (NO2); Calcd. for C14H20NO7P: C 48.70, H 5.84, N 4.05; Found: C 48.85, H 5.98, N 4.26.
O-methyl methyl[1-(2-nitrophenoxyacetoxy)propyl]phosphinate (7q) Yellow oil: 1H NMR (CDCl3, 200 MHz): δ=0.95 (t, 3H, –CH3, J=15 Hz), 1.40 (dd, 3H, –CH3, J=4, 15 Hz), 1.64, 2.04 (m, 2H, CH2), 3.66 (dd, 3H, –OCH3, J=12, 14 Hz), 4.84 (s, 2H, CH2), 5.04–5.34 (m, 1H, CH), 6.92–7.85 (m, 4H, Ar-H); 31P NMR (CDCl3, 81 MHz): δ=48.76, 50.39; IR (ν, cm−1): 3078 (Ph-H), 1755 (C=O), 1300, 876 (P–C), 1216, 1092 (C–O–C), 1185(P=O), 1039 (P–O–C), 1522, 1351 (NO2); Calcd. for C13H18NO7P: C 47.13, H 5.48, N 4.23; Found: C 46.89, H 5.44, N 4.31.
O-methyl methyl[1-(2-nitrophenoxyacetoxy)ethyl]phosphinate (7r) Brown oil: 1H NMR (CDCl3, 200 MHz): δ=1.45 (dd, 3H, –CH3, J=4, 15 Hz), 1.45–1.57 (m, 3H, –CH3), 3.73 (dd, 3H, –OCH3, J=12, 14 Hz), 4.85 (s, 2H, CH2), 5.27–5.48 (m, 1H, CH), 6.99–7.90 (m, 4H, Ar-H); IR (ν, cm−1): 3080 (Ph-H), 1764 (C=O), 1304, 893 (P–C), 1097 (C–O–C), 1191 (P=O), 1036 (P–O–C), 1524, 1353 (NO2); Calcd. for C12H16NO7P: C 45.43, H 5.08, N 4.41; Found: C 45.30, H 5.20, N 4.65.
O-methyl [(4-chlorophenyl)[3-(trifluoromethyl)phenoxyacetoxy]methyl](methyl)phosphinate (7s) Brown oil: 1H NMR (CDCl3, 200 MHz): δ=1.42 (dd, 3H, –CH3, J=4, 15 Hz), 3.67 (dd, 3H, –OCH3, J=12, 14 Hz), 4.84 (s, 2H, CH2), 6.18 (dd, 1H, CH, J=12, 14 Hz), 6.71–7.46 (m, 8H, Ar-H); MS m/z (%): 436 (M+ 19.82), 239 (100.00), 175 (63.87), 94 (52.68), 77 (50.94); IR (ν, cm−1): 3076 (Ph-H), 1770 (C=O), 1302, 887 (P–C), 1213, 1087 (C–O–C), 1168 (P=O), 1126 (C–O), 1042 (P–O–C), 1329 (C–CF3); Calcd. for C18H17ClF3O5P: C 49.49, H 3.92; Found: C 49.35, H 4.10.
O-methyl methyl[(3-nitrophenyl)[3-(trifluoromethyl)phenoxyacetoxy]methyl]phosphinate (7t) Brown oil: 1H NMR (CDCl3, 200 MHz): δ=1.46 (dd, 3H, –CH3, J=4, 15 Hz), 3.70 (dd, 3H, –OCH3, J=12, 14 Hz), 5.06 (s, 2H, CH2), 6.32 (dd, 1H, CH, J=12, 14 Hz), 7.01–8.35 (m, 8H, Ar-H); MS m/z (%): 447 (M+ 38.65), 239 (96.66), 175 (44.68), 145 (45.10), 93 (100).00; IR (ν, cm−1): 3086 (Ph-H), 1773 (C=O), 1304, 876 (P–C), 1214, 1081 (C–O–C), 1168 (P=O), 1125 (C–O), 1040 (P–O–C), 1329 (C–CF3); Calcd. for C18H17F3NO7P: C 48.33, H 3.83, N 3.13; Found: C 48.32, H 4.01, N 3.17.
O-methyl methyl[1-[3-(trifluoromethyl)phenoxyacetoxy]propyl]phosphinate (7u) Yellow liquid: 1H NMR (CDCl3, 200 MHz): δ=0.99 (t, 3H, –CH3, J=7 Hz), 1.41 (t, 3H, –CH3, J=13 Hz), 1.73–2.02 (m, 2H, CH2), 3.72 (dd, 3H, –OCH3, J=11 Hz), 4.78 (s, 2H, CH2), 5.23–5.28 (m, 1H, CH), 7.11–7.46 (m, 4H, Ar-H); IR (ν, cm−1): 3079 (Ph-H), 1764 (C=O), 1303, 873 (P–C), 1216, 1083 (C–O–C), 1178 (P=O), 1125 (C–O), 1041 (P–O–C), 1330 (C–CF3); Calcd. for C14H18F3O5P: C 47.46, H 5.12; Found: C 47.32, H 5.25.
O-methyl methyl[1-[3-(trifluoromethyl)phenoxyacetoxy]ethyl]phosphinate (7v) Yellow liquid: 1H NMR (CDCl3, 200 MHz): δ=1.42 (dd, 3H, –CH3, J=12, 14 Hz), 1.45–1.55 (m, 3H, –CH3), 1.73–2.02 (m, 2H, CH2), 3.72 (dd, 3H, –OCH3, J=10, 12 Hz), 4.73 (s, 2H, CH2), 5.27–5.48 (m, 1H, CH), 7.08–7.46 (m, 4H, Ar-H); IR (ν, cm−1): 3080 (Ph-H), 1764 (C=O), 1305, 890 (P–C), 1218, 1081 (C–O–C), 1181 (P=O), 1125 (C–O), 1036 (P–O–C), 1330 (C–CF3); Calcd. for C13H16F3O5P: C 45.89, H 4.74; Found: C 45.71, H 4.72.
O-methyl [(4-chlorophenyl)(2-chloro-4-nitrophenoxyacetoxy)methyl](methyl)phosphinate (7w) Yellow solid: 1H NMR (CDCl3, 200 MHz): δ=1.41 (dd, 3H, –CH3, J=12, 14 Hz), 3.67 (dd, 3H, –OCH3, J=11, 12 Hz), 4.99 (s, 2H, CH2), 6.20 (t, 1H, CH, J=5 Hz), 6.90–8.33 (m, 7H, Ar-H); MS m/z (%): 447 (M+ 9.94), 244 (100.00), 233 (72.64), 186 (85.68), 93 (71.00); IR (ν, cm−1): 3106, 3043 (Ph-H), 1768 (C=O), 1288, 894 (P–C), 1235, 1075 (C–O–C), 1180 (P=O), 1126 (C–O), 1040 (P–O–C), 1515, 1345 (NO2); Calcd. for C17H16Cl2NO7P: C 45.55, H 3.60, N 3.12; Found: C 45.65, H 3.85, N 3.12.
O-methyl [(2-chloro-4-nitrophenoxyacetoxy)(3-nitrophenyl)methyl](methyl)phosphinate (7x) Yellow solid: 1H NMR (CDCl3, 200 MHz): δ=1.46 (dd, 3H, –CH3, J=12, 14 Hz), 3.69 (dd, 3H, –OCH3, J=11, 12 Hz), 5.06 (s, 2H, CH2), 6.32 (dd, 1H, CH, J=12, 14 Hz), 6.94–8.35 (m, 7H, Ar-H); MS m/z (%): 458 (M+ 20.51), 244 (86.64), 228 (34.2), 186 (39.58), 93 (100.00); IR (ν, cm−1): 3087, 3043 (Ph-H), 1775 (C=O), 1288, 877 (P–C), 1228, 1075 (C–O–C), 1171 (P=O), 1126 (C–O), 1032 (P–O–C), 1524, 1341 (NO2); Calcd. for C17H16ClN2O9P: C 44.50, H 3.52, N 6.10; Found: C 44.51, H 3.65, N 6.22.
O-methyl [1-(2-chloro-4-nitrophenoxyacetoxy)propyl](methyl)phosphinate (7y) Yellow oil: 1H NMR (CDCl3, 200 MHz): δ=1.03 (t, 3H, –CH3, J=7 Hz), 1.48 (dd, 3H, –CH3, J=12, 14 Hz), 1.54–2.04 (m, 2H, CH2), 3.75 (dd, 3H, –OCH3, J=11 Hz), 4.94 (s, 2H, CH2), 5.25–5.32 (m, 2H, CH2), 6.98 (dd, 1H, CH, J=12, 14 Hz), 8.15–8.42 (m, 3H, Ar-H); IR (ν, cm−1): 3107, 3048 (Ph-H), 1764 (C=O), 1289, 893 (P–C), 1075 (C–O–C), 1191 (P=O), 1126 (C–O), 1039 (P–O–C), 1515, 1347 (NO2); Calcd. for C13H17ClNO7P: C 42.69, H 4.68, N 3.83; Found: C 42.69, H 4.68, N 3.75.
O-methyl [1-(2-chloro-4-nitrophenoxyacetoxy)ethyl](methyl)phosphinate (7z) Yellow oil: 1H NMR (CDCl3, 200 MHz): δ=1.42 (dd, 3H, –CH3, J=12, 14 Hz), 1.47–1.58 (m, 3H, –CH3), 3.75 (dd, 3H, –OCH3, J=10, 12 Hz), 4.90 (s, 2H, CH2), 5.27–5.75 (m, 1H, CH), 6.89–8.31 (m, 3H, Ar-H); IR (ν, cm−1): 3109 (Ph-H), 1764 (C=O), 1289, 894 (P–C), 1215, 1075 (C–O–C), 1193 (P=O), 1126 (C–O), 1036 (P–O–C), 1516, 1346 (NO2); Calcd. for C12H15ClNO7P: C 40.98, H 4.30, N 3.98; Found: C 40.68, H 4.30, N 4.03.
O-methyl methylphosphinate 1 was synthesized in a two-step sequence starting from phosphorus trichloride, methyl iodide and aluminium trichloride according to the literature.11,12) O-methyl 1-hydroxyalkylphosphinates 3 were prepared by adding 1 and several kinds of aldehydes 2 using triethylamine as a catalyst to produce yields of 70–90%. Different phenoxyacetic acids 5 were prepared in satisfactory yields by the reaction of corresponding phenols with ethyl bromoacetate in the presence of K2CO3 in DMSO followed by hydrolysis; phenoxyacetyl chlorides 6 could be easily obtained by treating compounds 5 with thionyl chloride.5,6) The title compounds 7 (Table 1) can be obtained by the condensation of compounds 3 with different phenoxyacetyl chlorides 6. The synthetic route is shown in Fig. 2.
The herbicidal activity of compounds 7 was evaluated at a rate of 150 g ai/ha. They were tested for pre-emergence and post-emergence inhibitory effect against Triticum aestivum L., Digitaria sanguinalis Scop, Echinochloa crusgalli Beava, Brassica napus L., and Cirsium japonicum DC. The in vivo herbicidal activities of compounds 7 were evaluated using a previously reported procedure.5) The percentage of growth inhibition of roots and aerial parts was calculated in relation to the mass of the roots and aerial parts of the control respectively. The compound I-1 was used as the standard of comparison for the activity of the title compounds 7.
As shown in Table 2, most of the compounds showed excellent herbicidal activities against dicotyledonous weeds (B. napus and C. japonicum); 7a–7g especially exhibited much higher herbicidal activity against C. japonicum in post-emergence treatment than in pre-emergence. As for monocotyledonous weeds, 7a–7m displayed more or similar herbicidal activities against D. sanguinalis and E. crusgalli in comparison with compound I-1. As for D. sanguinalis, most of the compounds with 2,4-diCl as X and Y (7b–7e, 7g–7i) exhibited much higher herbicidal activity in post-emergence treatment than in pre-emergence, whereas some compounds, especially 7e and 7i, showed much higher herbicidal activity against E. crusgalli in pre-emergence treatment than in post-emergence. As for monocotyledonous crop T. aestivum (wheat), most of compounds 7 showed weaker herbicidal activities as compared with compound I-1. This result suggests that compounds 7 have a higher level of selectivity for wheat, one of China’s main crops.
| Compd. | Tria | Diga | Echa | Braa | Cira | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preb | Postb | Preb | Postb | Preb | Postb | Preb | Postb | Preb | Postb | |
| 7a | 37 | 35 | 100 | 100 | 70 | 76 | 99 | 100 | 36 | 100 |
| 7b | 8 | 27 | 99 | 100 | 75 | 73 | 100 | 100 | 55 | 100 |
| 7c | 24 | 35 | 98 | 100 | 90 | 76 | 100 | 100 | 100 | 100 |
| 7d | 48 | 24 | 17 | 100 | 75 | 46 | 100 | 100 | −9 | 100 |
| 7e | 43 | 16 | 83 | 100 | 90 | 19 | 100 | 100 | 18 | 100 |
| 7f | 49 | 49 | 100 | 100 | 90 | 97 | 100 | 100 | 91 | 100 |
| 7g | 29 | 41 | 83 | 100 | 90 | 95 | 100 | 100 | 55 | 100 |
| 7h | 35 | 37 | 17 | 100 | 85 | 62 | 100 | 100 | 9 | 0 |
| 7i | 28 | 29 | 83 | 100 | 100 | 65 | 100 | 100 | 99 | 93 |
| 7j | −10 | 12 | −17 | 0 | −15 | −5 | −6 | 0 | 9 | 0 |
| 7k | 16 | 14 | 17 | 29 | 25 | 24 | 13 | 5 | 0 | 7 |
| 7l | 21 | 29 | −17 | 0 | −20 | −3 | 14 | −2 | 9 | 93 |
| 7m | 21 | 9 | 0 | — | 10 | 30 | −24 | 24 | −9 | 21 |
| 7n | 2 | −1 | −17 | 29 | 25 | 14 | −37 | −15 | 18 | — |
| 7o | 7 | 10 | 0 | 0 | −5 | 27 | 7 | 22 | 0 | 0 |
| 7p | 23 | 10 | 0 | 0 | 5 | 2.7 | −2.8 | 0.0 | 36.4 | — |
| 7q | 23 | 16 | −17 | 29 | 20 | 19 | −11 | −9 | −18 | 0 |
| 7r | 11 | 10 | 0 | 29 | 25 | 5 | 21 | 6 | 9 | 29 |
| 7s | 60 | 46 | 100 | 100 | 95 | 100 | 100 | 100 | 91 | 93 |
| 7t | 35 | 23 | 100 | 100 | 50 | 49 | 100 | 100 | 91 | 100 |
| 7u | 52 | 26 | 100 | 100 | 55 | 100 | 100 | 100 | 100 | 86 |
| 7v | 48 | 16 | 100 | 100 | 75 | 60 | 100 | 100 | 100 | 100 |
| 7w | 3 | −13 | 33 | 29 | −15 | 5 | −1 | −22 | −36 | 43 |
| 7x | 17 | 12 | 0 | 29 | −20 | 0 | −11 | −16 | 36 | 36 |
| 7y | 6 | 11 | −17 | 57 | 5 | −3 | 9 | 9 | 27 | 43 |
| 7z | −5 | −5 | 80 | 14 | 40 | −5 | 4 | 10 | −9 | 14 |
| I-1 | 93 | 37 | 70 | 55 | 70 | 53 | 100 | 100 | — | — |
a Tri, Triticum aestivum L.; Dig, Digitaria sanguinalis Scop; Ech, Echinochloa crusgalli Beava; Bra, Brassica napus L.; Cir, Cirsium japonicum DC. b Pre, pre-emergence treatment; post, post-emergence treatment.
Structure-activity relationship analysis indicates that the structure of substituents X and Y in a benzene ring has great influence on herbicidal activity. Compounds 7a–7i that have 2,4-diCl as X and Y showed higher herbicidal activities as compared with the compounds with 2-NO2 substitution, 7j–7r, that are almost inactive. Introducting 2-Cl–4-NO2 as X and Y resulted in compounds 7w–7z, which showed greatly decreased herbicidal activity as compared with 7d, 7f, 7h, and 7i. In addition, compounds 7s–7v that have 3-CF3 on the phenoxy-benzene ring showed good herbicidal activities, even more than those of compounds 7a–7i.
A series of O-methyl methyl[1-(substituted phenoxyacetoxy)alkyl]phosphinates was synthesized based on the synthetic modifications of structure I, and their herbicidal activity against five species was evaluated. SAR analyses indicated that the replacement of the phosphonate moiety in structure I with the phosphinate group had a favorable effect on herbicidal activity and a higher selectivity between monocotyledon crops and weeds. Our results showed these compounds 7 could be potential and selective herbicides for further development.
We gratefully acknowledge financial support of this work by the National Basic Research Program of China (2010CB126100), the National Key Technologies R & D Progaram of China (No. 2011BAE06B03), and the National Natural Science Foundation of China (No. 21172090). The research was supported in part by the PCSIRT (No. IRT0953).