Introduction
Currently, oximes and their derivatives have played a very important role in the molecular design and synthesis of modern medicines and pesticides.1–4) In particular, they have attracted considerable attention for decades because of their unique mode of action and low toxicity to non-target organisms.5) Containing a highly efficient pharmacophore of C=N–O, oximes have been found to exhibit excellent bioactivities; their excellent antitumor, antiviral, antiproliferative and antifungicidal activities have already been recognized and utilized by medicinal chemists and pharmaceutical scientists.6–9) In agricultural science, novel derivatives of strobilurins, benzoylphenylureas, and numerous thiazole and pyrazole derivatives that contain an oxime ether moiety possess a wide range of bioactivities.10–12)
Fragment-based lead discovery is an efficient method for screening low molecular weight compounds for new precursors.13) As a common substituent, phenyl and halophenyl rings can be found in many reported compounds that have different bioactivities.14,15) Different functional groups have different electronic effects on the phenyl rings of the drugs, and their substitution number and position may also affect the steric characteristics and the hydrophobicity of the designed molecule.16,17)
A series of general structures reported by Ke et al. showed high larvicidal activity against pests such as Myzus persicae.18) Furthermore, aiming to research fungicidal activity and to find better lead structure for this kind of compound, we designed and synthesized compounds B1–B25 shown in Fig. 1. Their structures and bioactivity data are shown in Table 1.
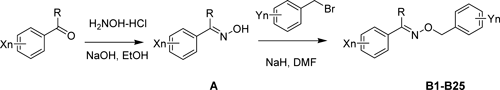
Fig. 1. Synthetic pathway of the target compounds B1–B25
Table 1. The structures, larvicidal and fungicidal activities of target compounds
B1–
B25
| Compd. |
R |
Xn |
Yn |
Myzus persicae
|
Rhizoctonia solani
|
Sclerotinia sclerotiorum
|
|
B1
|
CH3 |
2,4-Cl2 |
2-Cl |
185.1 (133.4–256.9) |
>100 (36.5)a |
>100 (21.8)a |
|
B2
|
CH3 |
2,4-Cl2 |
3-Cl |
>200 (29.6%)a |
>100 (36.7)a36.7 |
>100 (17.7)a |
|
B3
|
CH3 |
2,4-Cl2 |
4-Cl |
207.8 (126.5–341.3) |
>100 (37.9)a |
>100 (14.3)a |
|
B4
|
CH3 |
2,4-Cl2 |
2-Br |
218.1 (168.4–282.6) |
57.4 (34.5–88.7) |
>100 (19.7)a |
|
B5
|
CH3 |
2,4-Cl2 |
3-Br |
>250 (29.6%)a |
>100 (29.9)a |
>100 (22.1)a |
|
B6
|
CH3 |
2,4-Cl2 |
4-Br |
92.8 (86.3–99.7) |
>100 (35.0)a |
>100 (26.5)a |
|
B7
|
CH3 |
2,4-Cl2 |
2-NO2 |
125.1 (93.3–167.6) |
>100 (35.6)a |
>100 (20.0)a |
|
B8
|
CH3 |
2,4-Cl2 |
3-NO2 |
>250 (42.4%)a |
48.7 (29.6–98.5) |
>100 (28.3)a |
|
B9
|
CH3 |
2,4-Cl2 |
4-NO2 |
>250 (45.6%)a |
67.7 (30.6–112.4) |
>100 (22.9)a |
|
B10
|
CH3 |
4-F |
4-Br |
116.5 (95.1–142.7) |
>100 (24.6)a |
>100 (17.0)a |
|
B11
|
H |
4-F |
4-Br |
123.1 (87.8–172.6) |
11.0 (6.1–19.9) |
>100 (35.1)a |
|
B12
|
CH3 |
4-Br |
4-Br |
>250 (28.7%)a |
>100 (18.3)a |
>100 (22.9)a |
|
B13
|
H |
4-Br |
4-Br |
>250 (40.4%)a |
>100 (17.7)a |
>100 (11.8)a |
|
B14
|
CH3 |
4-Cl |
4-Br |
59.1 (43.4–80.6) |
>100 (37.3)a |
>100 (18.1)a |
|
B15
|
H |
3-Cl |
4-Br |
>250 (47.8%)a |
81.6 (51.0–130.6) |
>100 (25.2)a |
|
B16
|
CH3 |
4-NO2 |
4-Br |
102.2 (81.2–128.6) |
>100 (35.0)a |
>100 (19.5)a |
|
B17
|
H |
2-NO2 |
4-Br |
74.0 (61.4–89.1) |
>100 (25.5)a |
>100 (13.2)a |
|
B18
|
CH3 |
4-OCH3 |
4-Br |
44.6 (39.8–49.6) |
>100 (29.0)a |
>100 (13.8)a |
|
B19
|
H |
2-CF3, 5-Cl |
4-Br |
48.3 (45.9–50.8) |
>100 (37.7)a |
>100 (14.1)a |
|
B20
|
H |
2-CH3, 3-F |
4-Br |
53.6 (45.6–63.0) |
14.0 (11.8–16.6) |
>100 (35.6)a |
|
B21
|
H |
2, 3-F2 |
4-Br |
>250 (25.3%)a |
8.5 (5.5–13.3) |
>100 (23.4)a |
|
B22
|
H |
2, 6-F2 |
4-Br |
56.7 (49.9–64.3) |
>100 (36.7)a |
>100 (14.3)a |
|
B23
|
H |
6-Cl, 2-F |
4-Br |
52.0 (48.0–56.4) |
11.9 (7.6–18.6) |
>100 (18.1)a |
|
B24
|
CH3 |
4-OCH3, H |
4-Br |
37.7 (25.2–56.9) |
>100 (29.0)a |
>100 (13.8)a |
|
B25
|
CH3 |
4-CH3, H |
4-Br |
82.1 (69.7–96.6) |
19.6 (17.2–22.3) |
>100 (27.9)a |
| Imidacloprid |
— |
— |
— |
1.2 (0.3–10.5) |
— |
— |
| Thio. M |
— |
— |
— |
— |
7.3 (5.5–9.7) |
8.2 (6.0–11.5) |
LD50: Concentration that caused median lethal; EC50: Concentration that inhibited mycelium growth by 50%; 95% CL: 95% confidence interval of LD50 and EC50; Thio. M.: Thiophanate-methyl; a): Mortality against Myzus. at 200 µg/mL concentration and inhibition ratio at 50 µg/mL concentration against Rhizoctonia and Sclerotinia.
Materials and Methods
1. Instruments
1H-NMR spectra were recorded on a Bruker DPX 300 spectrometer using CDCl3 as the solvent and tetramethylsilane as the internal standard. Chemical shift values (δ) are given in parts per million. The melting points were determined using an X-4 Melting-point Apparatus with binocular microscope and are uncorrected. All reagents were of analytical reagent grade or chemically pure and were used without further purification. Silica gel (300–400 mesh) was used for column chromatography. Yields were not optimized.
2. Chemical synthesis
2.1. General synthetic procedure for the intermediate oxime (E)-2,4-dichlorophenyl ethanone oxime (A1)
A series of intermediate oximes were prepared according to the literature19) (Fig. 1). A mixture of 2′,4′-dichloroacetophenone (21.1 g, 112 mmol) and hydroxylamine hydrochloride (8.74 g, 168 mmol) in anhydrous ethanol (51.5 g) and water (3.68 g) was heated to 50°C in an oil bath under stirring, and sodium hydroxide (8.96 g, 224 mmol) was then added dropwise. The reaction mixture was further stirred at 70°C for 30 min, and the residue was concentrated by rotary evaporation to obtain (E)-2,4-dichlorophenyl ethanone oxime (A1) as a white solid.
The following series of compounds A2–A25 were synthesized by the same procedure.
2.2. General synthetic procedure for (E)-1-(2,4-dichlorophenyl)ethanone O-(2-chlorobenzyl) oxime (B1)
A 100-mL round-bottomed flask in an ice bath and equipped with a magnetic stirrer was charged with A1 (3.71 g, 10 mmol), dimethylformamide (DMF) (20 mL) and sodium hydride (50%, 0.58 g, 12 mmol). Then, a solution of 1-(bromomethyl)-2-chlorobenzene (2.98 g, 12 mmol) in DMF was added dropwise and the mixture was then stirred at room temperature for 7 hr. The mixture was then extracted with diethyl ether. The residue was purified by flash chromatography (PE/EA=8/1) to afford target compound B1 as a pale yellow solid.
(E)-1-(2,4-dichlorophenyl)ethanone O-(2-chlorobenzyl) oxime (B1) Yield: 53.1%. m.p.: 49–50°C. 1H-NMR (CDCl3/TMS) (δ): 2.24 (s, 3H, CH3), 5.18 (s, 2H, OCH2), 7.11 (s, 1H), 7.23–7.31 (m, 3H), 7.36–7.45 (m, 3H). Anal. Found: C, 54.82; H, 3.68; N, 4.26%. Calcd. for C15H12ONCl3: C, 54.77; H, 3.56; N, 4.25%.
Other substituted analogs were synthesized by the same method. Yields were between 34.2 and 76.5%. The analytical values for C, H, and N agreed with the calculated values within 0.3%. The 1H-NMR data are shown in Supplemental Table S1.
3. Biological assay
3.1. Biological assay of fungicidal activities
The fungicidal activities against Rhizoctonia solani and Sclerotinia sclerotiorum were tested in vitro using the mycelium growth rate test.20) The solidified plates containing 50 µg/mL test compounds were incubated with a 7 mm mycelium disk at 28°C. The mycelial elongation radius (mm) of the fungus settlements was measured after incubation for 48 hr. Thiophanate-methyl was used as the control at the same concentration and under the same conditions. Growth inhibition rates were calculated with the following equation: I=[(C−T)/C]×100, where I is the growth inhibition rate (%), C is the blank control settlement radius (mm), and T is the treatment group fungus settlement radius (mm). Lastly, the effective concentrations (EC50) of compounds B11, B20, B21, B23, B25 and Thiophanate-methyl that inhibited mycelium growth by 50% were obtained according to Finney’s probability value method.
3.2. Biological assay of larvicidal activities
The larvicidal activities of compounds B1–B25 against Myzus persicae were tested according to the reported procedure.21) The target compounds were dissolved in DMSO to a concentration of 200 µg/mL. Peach leaves with 60–100 Myzus persicae were dipped in the diluted solutions of the chemicals for 5 sec and then kept in the conditioned room for normal cultivation. Mortality was evaluated by determining the number of live larvae after 48 hr relative to the control using imidacloprid at the same concentration and with the same conditions.
Results and Discussion
1. Synthesis
In the first synthetic step, acetophenone and benzaldehyde containing different substituent groups were used as the primary reactants. The intermediate oximes A were easily prepared in good yields (>95%) under vigorous stirring for 30 min at 70°C and were used directly in the next step without further purification. Then, the target compounds B1–B25 were prepared by reacting the intermediate oximes with the substituted benzyl bromide at room temperature. Compound B17 had the lowest yield of 34.2%. The unoptimized yields of all B compounds ranged from 34.2 to 76.5%. The structures of all target compounds were well characterized by 1H-NMR and elemental analyses.
2. Biological assay
2.1. Fungicidal activities
As shown in Table 1, preliminary bioassays suggest that all compounds have some fungicidal activity against Rhizoctonia solani and Sclerotinia sclerotiorum, although none of them was better than Thiophanate-methyl. B11, B20, B21, B23 and B25 exhibited high fungicidal activities against Rhizoctonia solani, whose EC50 values ranged from 8.5 to 19.6 µg/mL. It may be deduced that fluorine may positively contribute to the fungicidal activity of these compounds because these five compounds all contain fluorine in their structure. In general, the data in Table 1 also show that the compounds have higher fungicidal activity against Rhizoctonia solani than against Sclerotinia sclerotiorum, for which the highest inhibition was only 35.1%.
2.2. Larvicidal activities
It can be seen in Table 1 that compounds B1–B25 showed fair to good larvicidal activity. Among them, the compounds B18–B20 and B22–B24 resulted in high larval mortality over 90% at 200 µg/mL, with LD50 values of 44.6, 48.3, 53.6, 56.7, 52.0 and 37.7 µg/mL, respectively. It may be deduced that steric effects and electronic effects may play a role in larvicidal activity, as compounds B18, B19 and B24 that have relatively large function groups –OCH3 and –CF3 gave relatively low LD50 values of 44.6, 48.3 and 37.7 µg/mL, respectively. As B6 showed better larvicidal activity than others in B1–B6, it may be deduced that the bromine atom, which was located at the para-position of the benzyl group was biologically superior to the chlorine atom. Study will be continued in the future.
Acknowledgment
This work was supported by the National Basic Research Program of China 2012CB114104, the Major State Basic Research Development Program of China (No. 2010CB126106) and the National High Technology Research and Development Program of China (2011BAE06B03).
References
- 1) J. X. Huang, Y. M. Jia, X. M. Liang, W. J. Zhu, J. J. Zhang, Y. H. Dong, H. Z. Yuan, S. H. Qi, J. P. Wu, F. H. Chen and D. Q. Wang: J. Agric. Food Chem. 55, 10857–10863 (2007).
- 2) B. A. Song, X. H. Liu, S. Yang, D. Y. Hu, L. H. Jin and Y. T. Zhang: Chin. J. Org. Chem. 25, 507–525 (2005).
- 3) M. Saad, G. Mrlina and J. P. Calmon: J. Agric. Food Chem. 40, 1249–1256 (1992).
- 4) D. Kubmarawa, J. T. Barminas and A. O. C. Aliyu: Arch. Appl. Sci. Res. 3, 131–138 (2011).
- 5) R. Sun, Y. Li, M. Lü, L. Xiong and Q. Wang: Bioorg. Med. Chem. Lett. 20, 4693–4699 (2010).
- 6) A. D. Pillai, P. D. Rathod, P. X. Franklin, H. Padh, K. K. Vasu and V. Sudarsanam: Biochem. Biophys. Res. Commun. 317, 1067–1074 (2004).
- 7) H. J. Park, K. Lee, S. J. Park, B. Ahn, J. C. Lee, H. Y. Cho and K. I. Lee: Bioorg. Med. Chem. Lett. 15, 3307–3312 (2005).
- 8) G. P. Ouyang, Z. Chen, X. J. Cai, B. A. Song, P. S. Bhadury, S. Yang, L. H. Jin, W. Xue, D. Y. Hu, S. Zeng and S. Zeng: Bioorg. Med. Chem. 16, 9699–9707 (2008).
- 9) G. Surkau, K. J. Böhm, K. Müller and H. Prinz: Eur. J. Med. Chem. 45, 3354–3364 (2010).
- 10) R. Sun, M. Lü, L. Chen, Q. Li, H. Song, F. Bi, R. Huang and Q. Wang: J. Agric. Food Chem. 56, 11376–11391 (2008).
- 11) S. Tu, L. H. Xu, L. Y. Ye, X. Wang, Y. Sha and Z. Y. Xiao: J. Agric. Food Chem. 56, 5247–5253 (2008).
- 12) H. Dai, Y. Q. Li, D. Du, X. Qin, X. Zhang, H. B. Yu and J. X. Fang: J. Agric. Food Chem. 56, 10805–10810 (2008).
- 13) K. Motoba, H. Nishizawa, T. Suzuki, H. Hamaguchi, M. Uchida and S. Funayama: Pestic. Biochem. Physiol. 67, 73–84 (2000).
- 14) R. A. E. Carr, M. Congreve, C. W. Murray and D. C. Rees: Drug Discov. Today 10, 987–992 (2005).
- 15) M. E. Y. Francisco, H. H. Seltzman, A. F. Gilliam, R. Á. Mitchell, S. L. Rider, R. G. Pertwee, L. A. Stevenson and B. F. Thomas: J. Med. Chem. 45, 2708–2719 (2002).
- 16) L. He, P. J. Gilligan, R. Zaczek, L. W. Fitzgerald, J. McElroy, H. S. Shen, J. A. Saye, N. H. Kalin, S. Shelton, D. Christ, G. Trainor and P. Hartig: J. Med. Chem. 43, 449–456 (2000).
- 17) S. Emami, M. Falahati, A. Banifatemi and A. Shafiee: Bioorg. Med. Chem. 12, 5881–5889 (2004).
- 18) S. Y. Ke, Z. G. Zhang, Z. W. Yang, K. M. Wang, Y. Liang, Y. N. Zhang, T. Long and A. B. Jiang: Chi. Pat, CN102030680A (2001).
- 19) A. Liu, X. Ou, M. Huang, X. Wang, X. Liu, Y. Wang, C. Chen and J. Yao: Pest Manag. Sci. 61, 166–170 (2005).
- 20) Y. P. Luo and G. F. Yang: Bioorg. Med. Chem. 15, 1716–1724 (2007).
- 21) J. B. Liu, W. F. Tao, Y. Hu, H. Dai and J. X. Fang: Chin. J. Org. Chem. 11, 1566–1570 (2006).


