2013 年 38 巻 2 号 p. 68-73
2013 年 38 巻 2 号 p. 68-73
Use of synthetic herbicides has been the most effective method for weed control in modern agriculture. However, researchers are also looking for alternative bioactive molecules for weed management due to the increasing number of herbicide-resistant weeds and growing concern regarding harmful effects of herbicides on the environment. Many higher plants produce various bioactive natural products that have the potential to inhibit growth of weeds. These bioactive natural products have also been suggested as future natural herbicides.1) However, the action mechanisms of many natural products are still poorly understood.
l-DOPA (l-3,4-dihydroxyphenylalanine) is a bioactive natural compound produced by a number of plant species, but velvet bean (Mucuna pruriens L., DC.) has high l-DOPA content (>5% in seeds).2,3) l-DOPA selectively inhibits the growth of many plant and weed species. l-DOPA (0.1 mM) suppressed root growth (>50%) of 24 plant species from 9 diverse families irrespective of monocotyledonous and dicotyledonous, broadleaf and narrowleaf, and C3 and C4.4,5) However, the mode of action of l-DOPA is not well elucidated in plants.
l-DOPA is oxidized to dopaquinone and dopaminequinone by polyphenol oxidase (PPO; EC 1.10.3.1, EC 1.14.18.1) and autoxidation, which generate reactive oxygen species (ROS, Fig. 1).6,7) Both ROS and quinones produced during l-DOPA oxidation may play a central role in its cytotoxicity. The ROS may damage the cellular machinery leading to programmed cell death.8) l-DOPA-induced growth inhibition in lettuce roots may be due to oxidative stress caused by ROS generated through oxidation of l-DOPA.9) However, l-DOPA did not enhance ROS production in soybean,10) thus plant species may differ in physiological responses to l-DOPA.

On the other hand, the quinones may covalently bind with sulfhydryl compounds such as protein amino acid cysteine to form quinoproteins.11) Quinoprotein formation may inhibit the normal functioning of cells through mitochondrial swelling and dysfunction, deactivation of enzymes, disrupting homeostasis, covalent modification of critical proteins and damage to DNA leading to apoptosis.12–14)
Previous in vitro studies have shown that dopaquinone and dopaminequinone, oxidation products of l-DOPA, may bind covalently with cysteine to produce quinoproteins.7) Furthermore, dopamine, which is a metabolite of l-DOPA, increased quinoproteins in animal and human cells causing cytotoxicity.12,15) Therefore, we hypothesize that cucumber root growth inhibition by l-DOPA may be due to quinoprotein formation through the binding of cysteine with dopaquinone and dopaminequinone. To the best of our knowledge, this is the first report to suggest quinoprotein formation by l-DOPA in plants and possible involvement of quinoproteins in the phytotoxic action of l-DOPA.
Seeds of cucumber (Cucumis sativus L., cv. Shimoshirazu-jibai) were germinated for 2 days in the dark. Two-day-old seedlings were transplanted in 0.5% agar containing 0, 0.05, 0.1, 0.5 or 1 mM l-DOPA (Wako Pure Chemical Industries, Ltd., Osaka, Japan) in plastic pots. Distilled water (0 mM l-DOPA) served as a control. These concentrations were selected after a survey of the literature.4,16,17) The pots were placed in a growth chamber at 25/20°C, for a 12/12 hr photoperiod, and with a photon flux density of 90–100 µmol/m2/sec. Root length was measured 3 days after transplanting and roots were harvested for further histochemical and biochemical analyses.
2. Cell viabilityEvans blue uptake and fluorescein diacetate (FDA)-propidium iodide (PI) staining were used to detect the death of cells.8) To determine Evans blue uptake, 1 cm roots were incubated in 0.25% (w/v) aqueous solution of Evans blue for 1 hr in the dark at 30°C. After 1 hr, the roots were washed with distilled H2O, and Evans blue was extracted with N,N-dimethylformamide for 24 hr in the dark at 30°C. Absorbance was measured at 600 nm using a spectrophotometer. Cell death was further assessed by staining the roots with a mixture of FDA and PI. One centimeter root segments were incubated with a mixture of FDA (12.5 µg/mL) and PI (5 µg/mL) at room temperature for 10 min in the dark followed by a washing of the roots with distilled H2O. The stained roots were observed and photographed using a fluorescence microscope (Nikon E600 with a B-2A filter, excitation 450–490 nm, emission≥520 nm; Nikon Corp., Tokyo, Japan).
3. Free cysteine and l-DOPA contentsFree cysteine and l-DOPA contents were determined as previously described18) with minor modifications. Roots (200 mg) were ground to a fine powder with liquid nitrogen and were homogenized in 1 mL of 15 mM HCl. The homogenate was centrifuged at 2,000×g for 2 min. The supernatant (0.5 mL) was deproteinized with 0.1 mL of 10% 5-sulfosalicylic acid for 15 min on ice. After centrifugation at 2,000×g for 15 min, the pH of the supernatant was adjusted to 2.2 with 1 N NaOH. The free cysteine and l-DOPA contents were determined by the ninhydrin colorimetric method using an automatic amino acid analyzer (JLC-500/V2, JEOL Ltd., Tokyo, Japan).
4. PPO assay and lipid peroxidationActivity of the PPO was assessed as previously described19) with minor modifications. Roots (75 mg) were ground to a fine powder using liquid nitrogen and were homogenized in 0.75 mL of 100 mM potassium phosphate buffer (pH 7) containing 1% polyvinylpolypyrrolidone (PVPP) and 0.1% Triton X-100. The homogenate was centrifuged at 15,000×g for 20 min, and the supernatant was used to determine the PPO activity. The reaction mixture of 1 mL contained 50 mM potassium phosphate buffer (pH 7) and 50 mM 4-methylcatechol as a substrate, and 100 µL crude extract was added to start the reaction. Absorbance was measured at 420 nm, and PPO activity was calculated using an extinction coefficient of 1433/M/cm.
Lipid peroxidation was detected by measuring thiobarbituric acid reactive substances (TBARS) as previously described.9) Roots (100 mg) were ground to a fine powder with liquid nitrogen and were homogenized in 1 mL of 0.1% trichloroacetic acid (TCA) followed by centrifugation at 10,000×g for 20 min. The supernatant (0.5 mL) was mixed with 1 mL 0.5% thiobarbituric acid (TBA) in 20% TCA. The mixture was placed in a heat bath at 98°C for 30 min. Thereafter, the mixture was cooled on ice for 5 min followed by centrifugation at 10,000×g for 5 min. The absorbance was measured at 532 nm and nonspecific absorbance at 600 nm was subtracted. An extinction coefficient of 155/mM/cm was used to calculate TBARS content.
5. Quinoprotein contentQuinoproteins were detected as previously described.15) Roots (75 mg) were ground to a fine powder with liquid nitrogen and were homogenized in 1 mL of 50 mM potassium phosphate buffer (pH 7.8) containing 1% PVPP, 1 mM EDTA-Na, 1 mM phenylmethylsulfonyl fluoride, and 0.1% Triton X-100 followed by centrifugation at 15,000×g for 15 min at 4°C. The supernatant was centrifuged again at 15,000×g for 5 min, and proteins in the supernatant were precipitated with 10% TCA for 30 min on ice. The precipitated proteins were collected by centrifugation at 15,000×g for 5 min, and the protein pellet was washed twice with acetone. Thereafter, a 0.45 mL denaturing buffer (6 M guanidine–HCl, 100 mM NaPO4, 10 mM Tris, pH 8.0) was added to the protein pellet followed by vortex mixing for 5 min. The protein mixture (0.40 mL containing 150 µg proteins) was added to 1 mL of 0.24 mM nitroblue tetrazolium in 2 M potassium glycinate (pH 10) followed by incubation at room temperature (28±1°C) for 1 hr in the dark. Absorbance was measured at 530 nm using a spectrophotometer.
6. Statistical analysisAll data are presented as means±standard error of three replications. All experiments were repeated at least once under identical conditions. The data were subjected to one-way ANOVA followed by Tukey’s HSD test to determine significant differences among the treatment means at p<0.05.
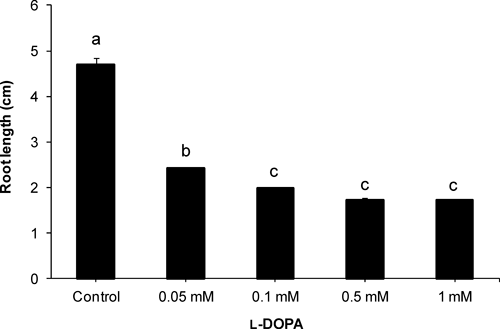
l-DOPA significantly inhibited root growth of cucumber (Fig. 2). At a lower concentration (0.05 mM), the root length of the cucumber was about 50% of control, which was further reduced to 42% of control at 0.1 mM l-DOPA. Though higher concentrations (0.5 and 1 mM) did not further decrease the root length significantly, roots were very weak and fragile at higher concentrations. Moreover, morphological changes were observed in the roots. l-DOPA treatment (0.1 and 1 mM) inhibited root hair formation (Fig. 4).
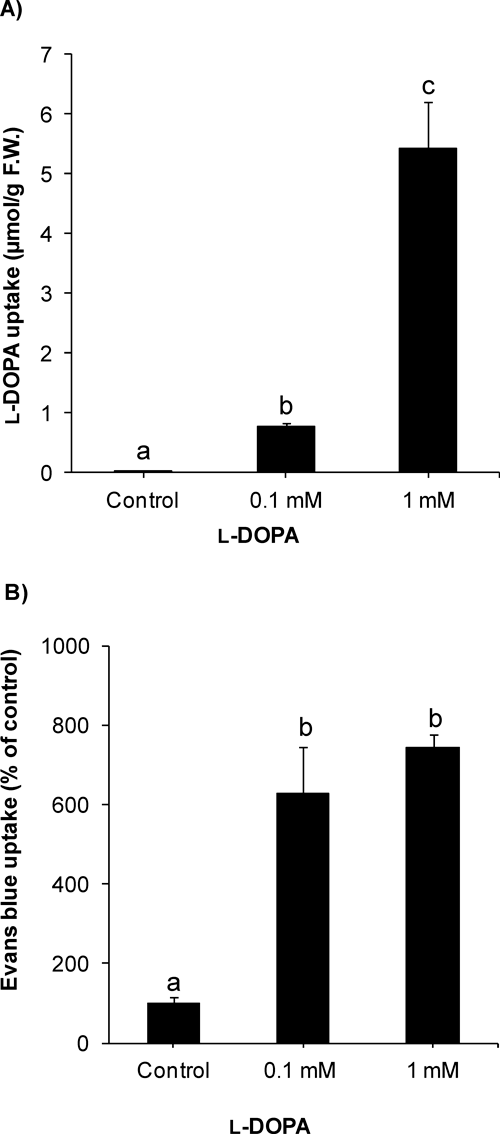
2. l-DOPA uptake and cell viabilityl-DOPA uptake was measured after the compound treatment in cucumber roots. Increase in l-DOPA content showed significant uptake by the roots (Fig. 3a). The l-DOPA uptake was increased in a concentration-dependent manner. Furthermore, histochemical staining was used to detect cell death. Evans blue uptake was significantly enhanced after l-DOPA treatment (Fig. 3b). At 0.1 mM l-DOPA, Evans blue content was >600% of control. Cell viability was also assessed using FDA-PI staining. Application of l-DOPA (0.1 and 1 mM) induced reddish fluorescence in cucumber roots, which indicated the death of cells while green fluorescence in control roots showed normal, alive cells (Fig. 4).

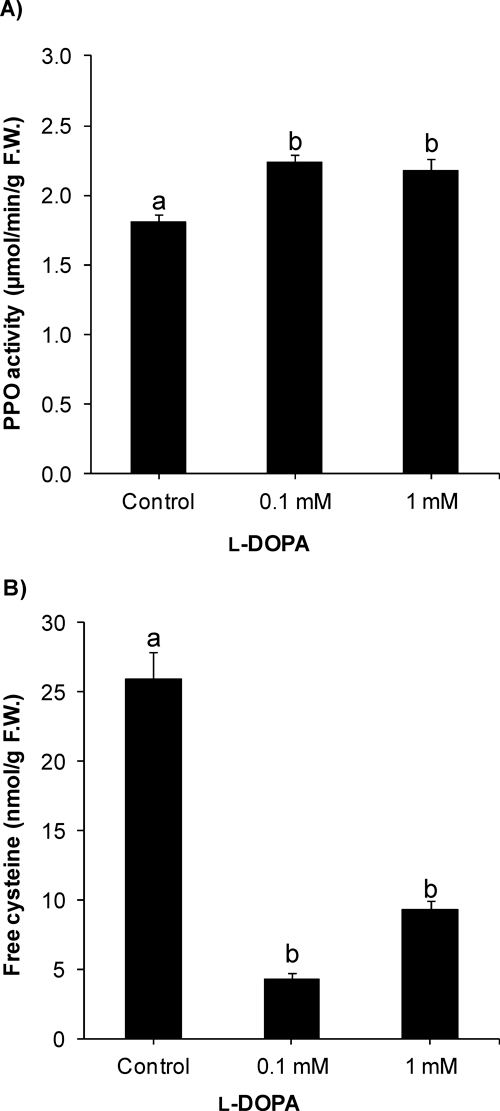
Activity of PPO was significantly increased by l-DOPA in cucumber roots (Fig. 5a). At 0.1 mM l-DOPA, PPO activity was 123% of control. However, there was no significant further change in PPO activity at a higher concentration (1 mM) of l-DOPA. On the other hand, free cysteine content was significantly decreased after treatment with l-DOPA (0.1 and 1 mM) (Fig. 5b). The free cysteine content was only 17% of control at 0.1 mM l-DOPA, which was not significantly affected using higher concentration of l-DOPA.
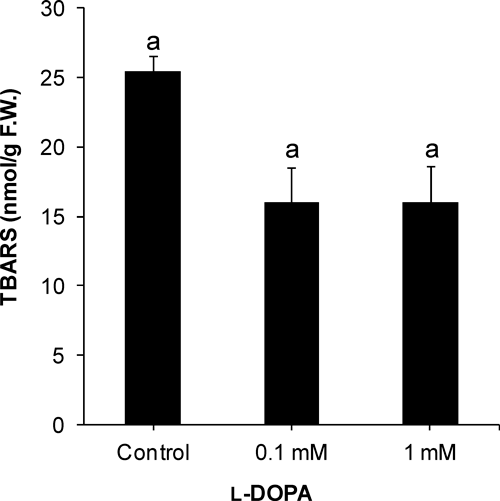
Lipid peroxidation was indicated by TBARS measurement, which was not significantly changed after l-DOPA treatment (Fig. 6). Further, quinoprotein formation was detected. l-DOPA (0.1 and 1 mM) markedly enhanced production of quinoproteins in cucumber roots (Fig. 7). Quinoprotein content was about fourfold of control at 0.1 mM l-DOPA, which increased further at 1 mM l-DOPA.

l-DOPA inhibits growth of several plant and weed species.4,5) Consistent with previous studies, l-DOPA significantly inhibited root growth of cucumber even at lower concentrations, 0.05 and 0.1 mM. The concentration-dependent increase in l-DOPA content in roots indicated that it was absorbed efficiently by the cucumber roots.
The phytotoxicity of l-DOPA was reported to be linked mainly with activity of PPO or autoxidation.6) PPO oxidizes l-DOPA to dopaquinone and dopaminequinone leading to melanin formation, and the oxidation process produces ROS.6,9) In the present study, we determined the activity of PPO, which was significantly enhanced after l-DOPA treatment. In plants, the main focus of PPO research has been on postharvest browning of fruits and vegetables.20) The increase in PPO activity in cucumber roots may be due to activation of PPO by l-DOPA. A recent study suggested that a single gene encodes the expression of PPO in cucumber.21) However, the precise mechanism of the increase in PPO activity in cucumber after l-DOPA treatment is unclear.
Increase in PPO activity strongly suggests that l-DOPA was actively oxidized to dopaquinone and dopaminequinone. However, autoxidation may also be involved in the oxidation process.22) The quinones may covalently bind with sulfhydryl compounds such as protein amino acid cysteine to form quinoproteins.7,11) We determined the free cysteine content in cucumber roots. l-DOPA treatment significantly reduced free cysteine content in the plant roots, which was in line with the significant increase in PPO activity. The cysteine might have been utilized in covalent binding with quinones to form quinoproteins. Further, quinoprotein formation was enhanced after l-DOPA treatment.
Production of quinoproteins may inhibit plant growth through cross linking of membrane proteins, deactivation of enzymes, mitochondrial dysfunction, disrupting homeostasis and DNA fragmentation leading to apoptosis.12–14,23) Previously, it has been reported that l-DOPA inhibited mitochondrial complex I (NADH dehydrogenase) and complex IV (cytochrome c oxidase) activities in rat and human mitochondria, respectively.24,25) Moreover, quinoproteins from dopaminequinone induced mitochondrial swelling and permeability transition of the mitochondrial membrane and reduced complex I activity and electron transport leading to dysfunction of mitochondria in human and rat brains.12,26,27) Recent proteomics studies using radiolabeled dopamine showed that dopaminequinone covalently modified critical proteins such as mitochondrial chaperonin, ubiquinol-cytochrome c reductase, mortalin, mitofilin, creatine kinase, and NADH dehydrogenase in animal and human cells.28,29) The covalent modification of these proteins by dopaminequinone may contribute to cytotoxicity.12,15)
l-DOPA itself is not toxic; only oxidation products of l-DOPA such as reactive oxygen species and reactive quinones are phytotoxic.6,26) In the present study, lipid peroxidation was not enhanced; therefore, the growth inhibition of cucumber roots may be due to quinoprotein formation. Previously, l-DOPA induced lipid peroxidation in lettuce roots, but it did not cause oxidative stress in soybean roots.9,10) l-DOPA did not increase lipid peroxidation in cucumber roots suggesting that oxidative stress may not be directly involved in action of l-DOPA in cucumber roots. That lipid peroxidation was not increased may be due to ROS scavenging by antioxidant enzymes and/or suppression of ROS generation by cysteinyldopa and quinoprotein formation through the binding of dopaquinone and dopaminequinone with cysteine.10,30,31)
Another research group hypothesized that l-DOPA inhibited growth of soybean may be by activation of a phenylalanine ammonia-lyase (PAL) and phenylpropanoid pathway thus accumulating phenolic compounds—including lignin—and increasing peroxidase activity.32) Though we do not rule out this possibility, it is unlikely because increase in PAL activity to accumulate phenylpropanoids is a general defense response of plants to biotic and abiotic stresses.33,34) Moreover, in that paper, a lower concentration (0.1 mM) of l-DOPA significantly enhanced peroxidase activity and phenolic content while there was no effect on soybean root growth at that concentration.32) Therefore, an increase in phenylpropanoids and lignin content might not be the primary cause of l-DOPA phytotoxicity but the secondary defense response of soybean plants to l-DOPA stress.
In conclusion, l-DOPA reduced free cysteine content and increased PPO activity and quinoprotein formation in cucumber roots, suggesting that the cysteine might bind to dopaquinone and dopaminequinone to form quinoproteins. The quinoproteins may be involved in the phytotoxic action of l-DOPA in cucumber roots.