2014 Volume 39 Issue 1 Pages 48-52
2014 Volume 39 Issue 1 Pages 48-52
In developed countries, alimentation problems are related to quality and safety rather than availability due to the high yields of modern intensive agriculture. In the last decades, organic agriculture has conquered an important market share based on consumers’ concerns about food quality and public interest in environmental protection. Organic agriculture is vaguely defined and differently regulated from one country to another, but it does not use synthesized substances1) nor genetically modified organisms. Organic agriculture is perceived by the public as being “intrinsically safe,” but many highly toxic compounds are natural and not artificial (botulinic toxin, ricin, strychnine, aflatoxin, anatoxin, microcistine, etc.). In fact, there is no scientific evidence to support the hypothesis that natural pest control products have a lower risk for the environment in comparison with synthesized insecticides.2) The list of compounds used as bioinsecticides is relatively short; the most used include rotenone, pyrethrum, azadirachtin, spinosyn, sabadilla and ryania.1) Among them, rotenone was proven to be a risk factor for Parkinson disease,3) and pyrethrum is suspected to be a carcinogen4); however, both are still used in organic agriculture because they have a natural origin. Despite recent improvement, biochemical pesticides are less effective in comparison with classical pesticides,5) and, consequently, larger doses or multiple treatments are required.6,7) Thus, food might be contaminated with higher concentrations of biochemical pesticides.
This paper focuses on the investigation of potential non-specific interactions between acetylcholinesterases (AChE, EC 3.1.1.7) and certain biochemical pesticides. AChE is a key enzyme in the nervous system that rapidly terminates nerve impulses by catalyzing the hydrolysis of the neurotransmitter acetylcholine, but it is involved in many other processes such as learning and memory8) or neurodegenerative conditions (i.e., Parkinson’s or Alzheimer’s disease).9) AChE is an allosteric enzyme that has in its structure a deep and narrow gorge leading to the active site, and inhibitors can bind with at least four regions: the esteratic part of the active site, the anionic part of the active site, the aromatic gorge, and the peripheral anionic site.10) Unlike synthetic neurotoxic insecticides from the organophosphate11) or carbamate classes, biochemical pesticides do not specifically target AChE. However, various non-specific interactions could occur between AChE and biochemical pesticides, and this enzyme is inhibited also by natural alkaloids and terpenoids.12) We have tested four bioinsecticides (spinosad, pyrethrum, neem bark extract, and veratrine) on three types of AChEs (one extracted from the electric eel and two from Drosophila melanogaster: the wild-type and a mutant made by site-directed mutagenesis in order to increase its sensitivity and its rate of phosphorylation or carbamoylation by organophosphates or carbamates).13) The structure of the Drosophila melanogaster enzyme is representative for insects,14) while the electric eel enzyme is representative for vertebrates.10) We have observed that spinosad does not have an inhibitory effect on the tested AChE, but pyrethrum, neem bark extract, and veratrine produce a dose-dependent inhibition.
Acetylcholinesterase (AChE, E.C. 3.1.1.7)-type V-S from Electric eel (Eel) provided by Sigma was dissolved in 0.05 M phosphate buffer saline (PBS) pH 7.0, and small aliquots were stored at −18°C. Two AChEs from wild-type (wt) and genetically modified (gm) Drosophila melanogaster (Dm)—produced,13) stabilized, and purified to a single protein by affinity chromatography as confirmed by electrophoresis15)—from Dr. Yvan Boublik (CRBM, Montpellier, France) were diluted with PBS pH 7.0 and stored at +4°C. Both AChE-Dm-wt and AChE-Dm-gm have been previously used for biosensor development16) and can be supplied for scientific testing (for inquiries, contact Professor Jean-Louis Marty: jlmarty@univ-perp.fr). The activity of the enzymes was spectrophotometrically measured using 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB, Ellman’s reagent) from Sigma; the working solution was prepared with 16 mg in 50 mL PBS. A stock solution of enzymatic substrate 0.05 M acetylthiocholine chloride (AcTCh) (Sigma) was prepared daily in distilled water and stored on ice. The biochemical pesticides used were supplied by Sigma: spinosad (producer code 33706), pyrethrum (producer code PS97), neem bark extract (producer code 73279) and veratrine (producer code V2379). Stock solutions of biochemical pesticides were prepared as follows: 10 mg spinosad in 5 mL methanol, 5 µL pyrethrum in 5 mL methanol, 100 µL neem bark extract diluted with 900 µL water, and 10 mg veratrine hydrochloride in 400 µL water and stored at +4°C.
2. Spectrophotometric measurementsSpectrophotometric measurements were carried out at λ=420 nm in a total volume of 1 mL using 60 mIU AChE per measurement in order to obtain an optimum analytical signal (absorbance). To 500 µL of Ellman reagent, we added PBS, 5–10 µL of enzyme working dilution (prepared daily), and 2–75 µL of biochemical pesticide dilutions. The enzyme was incubated with the biochemical pesticide for different time periods (usually 1 min). Subsequently, 10-40 µL of AcTCh was added, and the absorbance variation was immediately measured using a HR 4000 spectrophotometer from Ocean Optics (USA). Every spectrophotometric measurement was carried out in triplicate. The study of the inhibition for each enzyme-biochemical pesticide couple was repeated at least five times by comparing the enzyme activity in the presence of different concentrations of biochemical pesticide with a suitable blank. Blank tests were carried out to confirm that the organic solvents used for preparation of the biochemical pesticide stock solutions do not inhibit the enzymes.
3. Data interpretationThe experimental data points (enzymatic reaction rate for different substrate and biochemical pesticide concentrations) were interpreted by non-linear regressions to calculate the apparent kinetic parameters. Lineweaver-Burk linearization was used only for visualization of the inhibition type (more details are presented in the electronic supplementary material).
The interactions between four biochemical pesticides (spinosad, pyrethrum, neem bark extract and veratrine) and the three acetylcholinesterases (AChE-Eel, AChE-Dm-wt and AChE-Dm-gm) were investigated. Thus, 12 pairs of AChE/biochemical pesticides were studied. From the overview presented in Table 1, it may be noticed that spinosad does not have any inhibitory effect, while the rest of the biochemical pesticides induce different types of inhibition. AChE-Eel is inhibited un-competitively by neem bark extract, the inhibition of the AChE-Dm-wt by pyrethrum is competitive and the other enzyme-biochemical pesticide inhibitions were non-competitive. Variation of the inhibition type for the same inhibitor in the function of the enzyme origin18) or mutations in the structure18) was previously reported. Each particular case will be detailed below.
| Biochemical pesticides | AChE-Eel | AChE-Dm-wt | AChE-Dm-gm |
|---|---|---|---|
| Spinosad | n.i. | n.i. | n.i. |
| Pyrethrum | 2.5 ppm (NC) | 10 ppm (NC) | 1 ppm (C) |
| Neem bark extract | 1/10,000 dil. (UC) | 3/10,000 dil. (NC) | 3/10,000 dil. (NC) |
| Veratrine | 10 ppm (NC) | 16 ppm (NC) | 38 ppm (C) |
Abbreviations, n.i.: no inhibition, C: competitive, NC: non-competitive, UC: uncompetitive.
We did not observe a significant inhibitory effect of spinosad on any of the three tested AChEs, even at concentrations of 100 ppm and 10 min of incubation. Higher concentrations were not tested in order to avoid non-specific AChE denaturation by the methanol used to prepare the spinosad stock solution. Our previous work demonstrates that it is possible to use methanol concentrations up to 5% (v/v) if the inhibition percentage is calculated by measuring the enzymatic activity in the presence of the same methanol concentration.19) Spinosad consists of a mixture of two compounds (Spinosyns A and D) that are biosynthesized by the Saccharopolyspora spinosa bacterium. Its toxicity is due to the persistent activation of nicotinic acetylcholine receptors (nAChR) at a different site from nicotine or neonicotinoids and also affects γ-aminobutyric acid (GABA) receptors.20) There are in vivo measurements that demonstrate a reduction in the AChE activity in different organisms treated with spinosad, such as honey bees (Apis mellifera), for concentrations higher than 2.5 ppm,21) or Nile tilapia fish (Oreochromis niloticus) at 25 ppm.22) Our experiments performed in vitro demonstrate that the observed reduction in the AChE activity in animals treated with spinosad is caused by a phenomenon that is different from enzyme inhibition. The nicotinic acetylcholine receptors (nAChR) affected by spinosad are also inhibited by some organophosphates. The main toxicity mechanism of organophosphate insecticides consists in AChE inhibition, but it was demonstrated that some organophosphates inhibit nAChRs more potently than AChEs in the following decreasing order: disulfoton>parathion-ethyl>parathion-methyl>fenthion. It is interesting to note that nAChRs are more affected in vitro by the “thio-” parent insecticide form,23) whereas AChE is more inhibited by the “oxon-” metabolites,24) a fact, that once again, underlines the differences between the interaction of the AChE inhibitors with AChE and nAChR, respectively.
2. PyrethrumWe have observed that pyrethrum induces a dose-related inhibition with variable types: competitive inhibition for AChE-Dm-gm and non-competitive inhibition for both AChE-Eel and AChE-Dm-wt. It was observed that the inhibition percentage does not increase significantly for enzymes-pyrethrum contact longer than one minute. The biochemical pesticide used in these experiments is a mixture of six natural compounds. From the measurements carried out with a mixture of inhibitors, it cannot be determined whether only one or several compounds have inhibitory effects or if there are synergistic effects. Consequently, it is not possible to calculate a valid enzyme inhibition constant (KI), and only apparent values may be obtained from data interpolation (KIapp). Data interpretation allows only identification of the enzyme inhibition type and the differences between the sensitivities of the tested enzymes toward pyrethrum. Besides the differences between the enzymes concerning the inhibition type (non-competitive and respectively competitive), the sensitivity toward pyrethrum also varies (Supplemental Fig. S1). Thus, the most sensitive enzyme was the genetically modified insect enzyme (AChE-Dm-gm) that is ten times more sensitive in comparison with the wild-type enzyme (AChE-Dm-wt), while AChE-Eel was four times more resistant in comparison with AChE-Dm-gm. The concentrations required to induce a 20% inhibition of the enzymatic activity were: 2 ppm for AChE-Dm-gm, 5 ppm for AChE-Eel, and 20 ppm for AChE-Dm-wt. The same order of sensitivity is obtained from the comparison of the KIapp obtained by non-linear fitting of the experimental data (Fig. 1).
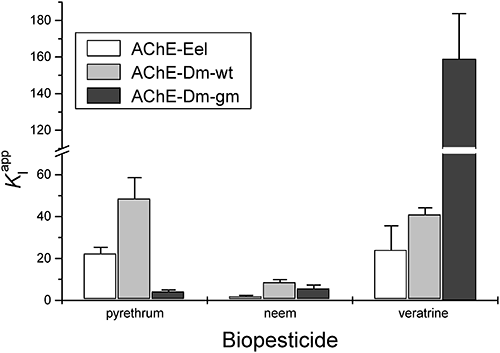
The main toxicity of both pyrethrin and pyrethroid insecticides is due to their binding on the sodium channel; insects may acquire resistance by point mutations in these channels.25) Other studies in vivo also demonstrate that the application of pyrethrum rapidly decreases the AChE activity in the central nervous system of Schizodactylus monstrosus,26) leading to an increased concentration of acetylcholine.27) AChE inhibition in vitro by permethrin (a synthetic pyrethroid insecticide with a similar chemical formula) was used to develop of a biosensor that had a detection limit of 3 ppm.28) It is interesting to note that, unlike some (carboxyl)esterases, AChE and butyrilcholinesterase (BuChE) do not have esterase activity on pyrethroid esters.29) Our study demonstrates that there is direct interaction between pyrethrum and AChE, and this may induce or accentuate some of the symptoms (e.g. impairment of the motor function or paresthesia) that are characteristic of this bioinsecticide.
3. Neem bark extractDifferent neem formulations, ranging from crude plant parts, seed oil, aqueous or non-aqueous solvent extracts, to purified bioactive ingredients are used and have variable efficiency or toxicity as insecticides or even “traditional treatments” of various ailments.28) Numerous components are found in neem extracts. We have used an aqueous extract from neem bark that contains propylene glycol because a water soluble formulation is necessary for in vitro measurement of enzymatic inhibition. The plant extract contains between 25 and 50% of different bark components, but the exact composition and concentrations of the compounds are not known (and probably vary between different batches); consequently, we express the quantity of inhibitor as the final dilution factor. Moreover, when using such complex bark extracts, it is impossible to determine whether the inhibition is due to a single or multiple compounds, and the test is able to evaluate only a global anti-cholinesterase activity. Our in vitro experiments demonstrated that the neem bark extract induces a dose-related inhibition of AChE with a different mechanism: uncompetitive for AChE-Eel and non-competitive for both AChE-Dm-wt and AChE-Dm-gm (Supplemental Fig. S2). The most sensitive enzyme was AChE-Dm-gm followed by both AChE-Eel and AChE-Dm-wt (Fig. 1). The dilution required to produce an inhibition of 20% was 0.1/1000 for AChE-Dm-gm and 0.3/1000 for both AChE-Eel and AChE-Dm-wt. Higher concentrations of bark extract lead to an increase of enzyme inhibition. Thus, for AChE-Eel, it was observed that a 1/1000 dilution is necessary to induce an inhibition of 35%, and a 3.5/1000 dilution is needed for 46% inhibition.
There are various neem extracts and formulations from Azadirachta indica with numerous active compounds that have anti-feeding or repellent effects, that induce growth disruption in insects,31) and that are toxic for fishes.32) There are studies that report that azadirachtin (the “principal” neem active ingredient) inhibits AChE activity of the brown planthopper (Nilaparvata lugens) at concentrations that induce significant mortality,33) and vepacide (an enriched neem oil-based preparation) inhibits AChE in the brain of rats.34) On the other hand, other studies indicate that neem extract does not inhibit AChE activity in the nervous system of cockroaches (Periplaneta americana), but induces excitatory action on the electrical activity by interfering with the ion channels from the nerve membrane.35) The differences between the results obtain from in vivo toxicological studies and in vitro enzymatic inhibition may be due to numerous processes that take place in living organism, such as adsorption of the toxins, their transport to the relevant organs, bioaccumulation, detoxification, differences between acute and long term effects, etc. The complex and variable composition of neem extracts is another factor because the components may have antagonist or synergetic effects. Our study indicates that neem extract has anticholinesterase activity, and, while this may not be relevant to some pest-control effects, it should be thoroughly tested before being used for medicinal purposes.
4. VeratrineVeratrine (aka sabadilla powder), a bioinsecticide that contains a mixture of several alkaloids extracted from the Liliaceae family, has a toxicity based on the opening of the voltage-dependent Na+ channels that, in turn, opens voltage-activated Ca2+ channels leading to the release of neurotransmitters.36) The calculated KIapp values are presented in Fig. 1. Our studies have demonstrated that veratrine produces a non-competitive inhibition of all tested AChEs (Supplemental Fig. S3). The most sensitive enzyme was AChE-Dm-wt, followed by AChE-Eel and AChE-Dm-gm. Thus, for 25 ppm veratrine, an inhibition of 39% was observed for AChE-Dm-wt, 30% for AChE-Eel, and only 15% for AChE-Dm-gm. The inhibition produced by veratrine is dose dependent; for example, the inhibition induced by 50 ppm veratrine on AChE-Dm-gm is 25% and by 75 ppm is 31%. Measurement of the enzyme inhibition was carried out after 1 min of incubation with veratrine, as it was observed that the inhibition degree did not increase with longer incubation times.
Several natural alkaloids are used for the treatment of Alzheimer’s disease based on their AChE inhibition (e.g. galanthamine, which induces in vitro a 50% inhibition at 0.9 ppm against AChE from the human brain).37) The non-competitive inhibitory effect of veratrine demonstrated in this study may be interesting for medical applications.
In this paper, we have investigated the interactions between four biochemical pesticides (spinosad, pyrethrum, neem bark extract and veratrine) and three acetylcholinesterases (one extracted from the electric eel (Electrophorus electricus) and two from the fruit fly (Drosophila melanogaster): the wild-type and a mutant. It was observed that spinosad does not have an anticholinesterase activity, while the other three biochemical pesticides induced a dose-dependent inhibition. Pyrethrum induced a competitive inhibition on AChE-Dm-gm, AChE-Eel was inhibited un-competitively by neem bark extract, and the other enzyme-biochemical pesticide inhibitions were non-competitive. The sensitivity of enzymes toward the biochemical pesticides was variable. Our results demonstrate that it is necessary to rigorously test the safety of biochemical pesticides and any natural bioactive compounds before every intended use.
This work was supported by Romanian National Authority for Scientific Research, CNCS–UEFISCDI (projects PN II-RU TE-100/2010 and PN II-RU TE-3-0076/2011) and Romania–France bilateral mobility grant Brancusi (project 487/2011). Paper revision by Dr. Alina Vasilescu is gratefully acknowledged.