2018 年 43 巻 4 号 p. 261-265
2018 年 43 巻 4 号 p. 261-265
A plant growth regulating agent “Fuji-one” has been used to control non-parasitic damping-off (Murenae disease) of rice seedlings. Its active ingredient, isoprothiolane (diisopropyl 1,3-dithiolan-2-ylidenemalonate, IPT), enhances root elongation of rice and Arabidopsis. To understand the mechanisms of IPT’s effect on root development, its effect on Arabidopsis root cells was investigated histologically. IPT at a lower concentration (12.5 µg/mL) had no effect on root cell elongation, whereas it enhanced cell division in the root meristem. Histological analysis using phytohormone-related mutants indicated that jasmonic acid and ethylene were involved in the enhanced cell division. In contrast, IPT at a higher concentration (75 µg/mL) suppressed both cell elongation and cell division, in which jasmonic acid and ethylene were not involved. In addition, root hair formation was suppressed by treatment with IPT. These analyses demonstrated that IPT (12.5 µg/mL) enhanced root elongation by activating cell division in a jasmonic acid- and ethylene-dependent manner.
Isoprothiolane (diisopropyl 1,3-dithiolan-2-ylidenemalonate, IPT) is an active ingredient of a rice blast controlling agent “Fuji-one” (Nihon Nohyaku Co., Ltd.)1,2) and has been used also as a plant growth regulating agent to protect rice seedlings cultured in nursery flats from non-parasitic damping-off (Murenae disease) (Fig. 1).3) A plausible mechanism for IPT’s plant growth regulating activity is acceleration of rice root development, which was observed at IPT concentrations as low as 6 µg/mL, however, the molecular mechanism remains to be clarified.4,5) Extensive studies on IPT’s plant growth regulating activity in rice have demonstrated various physiological effects on rice; the promotion of auxin activity in lamina inclination assay,6) the induction of ethylene (ET) production in the seedlings and callus,6) the enhancement of acid phosphatase activity in the root,7) the upregulation of methionine sulfoxide reduction leading to ET production,8) and the promotion of water permeability of the root cell membrane.5) However, without genetic studies in rice, the contribution of these physiological changes to enhancement of root development cannot been determined.

Recently, a more detailed analysis using Arabidopsis indicated that IPT possesses dual activities for the enhancement and suppression of primary root elongation at a lower concentration (12.5 µg/mL) and at a higher concentration (75 µg/mL), respectively, in Arabidopsis as well as in rice.9) Furthermore, analyses using phytohormone-related Arabidopsis mutants and phytohormone biosynthesis inhibitors demonstrated that auxin-, jasmonic acid (JA)- and ET-mediated signals are involved in the positive effect of a lower IPT concentration.9) During plant growth under normal conditions, endogenous auxin is known to function in root elongation by activating cell division in the root apical meristem,10) whereas JA and ET, important phytohormones functioning in stress responses, are known to suppress root elongation.11,12) Although the roles of these phytohormones in the plant growth regulating activity of IPT are important, the question has been raised as to how JA and ET function in root elongation. Thus, the present investigation was conducted to clarify the histological effect of IPT on Arabidopsis root growth in relation to JA and ET signals. Here we report the characterization of IPT’s effects on the cell length, cell division, and formation of root hairs in the primary root.
Surface-sterilized Arabidopsis seeds were placed on 1/2 Murashige and Skoog (MS) (Duchefa Biochemie) agar plates (containing vitamins, 1% sucrose, and 0.8% agar) (140×100×14.5 mm) and incubated in a vertical position under a constitutive light condition and a temperature of 22°C for 2 days. Seedlings were transferred to fresh 1/2MS agar plates or 1/2MS agar plates supplemented with IPT (12.5 or 75 µg/mL) and incubated in a vertical position under the same condition.
2. Cell length measurementMicroscopic analysis of cell length in differentiation zone was performed with 7-day-old seedlings using a light microscope BX51 (Olympus) with an UPLanFLN 20X (NA 0.50) lens (Olympus) and a DP73 digital camera (Olympus). To compare the cell length between treatments, the trichoblast cell that shifted from the elongation step to the differentiation step was chosen as reliable estimate of the actual extent of cell elongation.13) From the images taken with the microscope system, the length of the first epidermal cell with a visible root hair bulge was measured using cellSens Standard 1.6 software (Olympus). The cell lengths of at least 20 different roots were measured for comparison and statistical analysis between treatments.
3. Quantitative analysis of root hairsRoot hairs in 7-day-old seedlings were viewed using a stereo microscope SZX12 (Olympus) with a DF PLAPO 1.2x PF (NA 0.11) lens (Olympus) and photographed using a DP73 digital camera (Olympus). The length of root hairs formed in a section between 1 to 6 mm from the first visible root hair bulge was measured using cellSens Standard 1.6 software (Olympus). Density was determined as the number of root hairs in the same section under a microscope. Data were taken from at least 4 different roots in each experiment.
4. Acetocarmine stainingRoots of 7-day-old seedlings were cut and incubated in acetocarmine solution, acc. to Kultschitzky (Tokyo Kasei) for 30 min at 78°C, followed by incubation in 0.24 N HCl in 20% methanol for 15 min at 57°C. The root samples were washed with 40%, 20%, and 10% ethanol for 15 min each at room temperature and then stored in 25% glycerol containing 5% ethanol till analysis. Stained root samples were viewed using a light microscope BX51 (Olympus) with an UPLanFLN 10X (NA 0.30) lens (Olympus) and photographed using a DP73 digital camera (Olympus). The stained meristem length was measured using cellSens Standard 1.6 software. Data were taken from at least 20 different roots in each experiment.
5. Propidium iodide stainingRoots of 5-day-old seedlings, 3 days after the IPT treatment, were cut and stained with 10 µg/mL propidium iodide (Nacalai) for 2 min, followed by rinsing in water to remove excess staining solution. Fluorescence in roots was detected using an LSM510 confocal laser scanning microscope (Zeiss) with a PlanAPOCHROMAT 20X objective lens (NA 0.8) (Zeiss). Propidium iodide was viewed at excitation wavelength of 543 nm and the fluorescence emission was collected between 608 and 629 nm. Cells of the root cortex layer were counted in a file from the quiescent center to the first elongated cell. Cell numbers from at least 6 different roots were counted for comparison and statistical analysis between treatments.
We have previously reported that IPT has dual activities on root elongation in Arabidopsis, namely the enhancement of primary root elongation at a lower concentration (12.5 µg/mL) and suppression at a higher concentration (75 µg/mL).9) Considering the root cell development through three morphologically distinguishable zones, the cell division zone in meristem, the elongation zone, and the differentiation zone, IPT probably influences cell proliferation or cell elongation in the root. To determine IPT’s effect on the primary root elongation, we first examined whether IPT influences the length of trichoblast cells in the lower part of the differentiation zone. After the end of elongation process, cell differentiation begins where the trichoblast cell forms a root hair.13,14) Microscopic analysis indicated that length of the first epidermal cell with a visible root hair bulge was not influenced by 12.5 µg/mL IPT but was significantly reduced by 75 µg/mL IPT (Fig. 2A, B). These results suggest that the negative effect of IPT on root elongation is due to the reduction in cell length, however, the positive effect is not due to the increase in cell size.
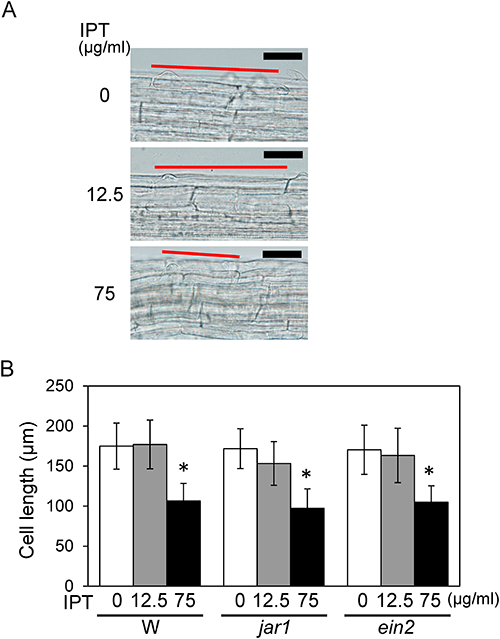
In a previous study, analyses using mutants and biosynthetic inhibitors revealed that JA and ET are involved in IPT’s positive effect on root elongation but not in its negative effect.9) To determine the involvement of these phytohormones in IPT’s effect on cell elongation, cell length was analyzed in jar1 and ein2 mutants defective in JA- and ET-signal transduction, respectively. Microscopic analysis of jar1 and ein2 mutants exhibited results similar to those for the wild-type (Fig. 2B). These results suggest that JA and ET are not involved in the suppressive effect of IPT on cell elongation process at a higher concentration.
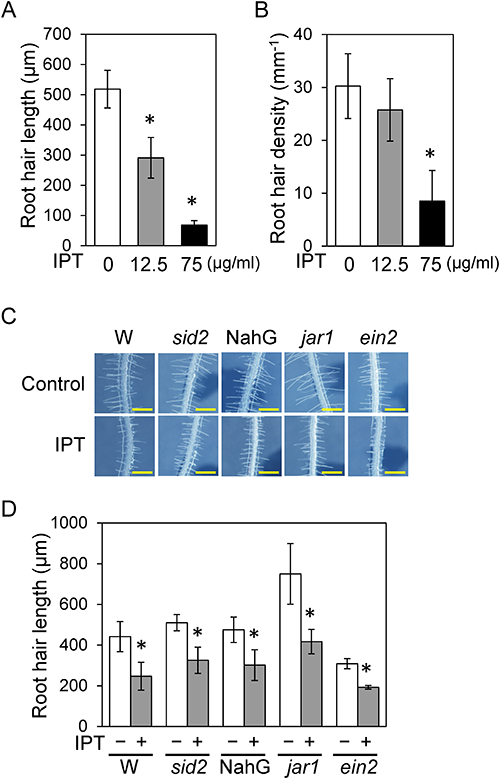
2. Effect of IPT on root hair formation in the differentiation zoneMicroscopic analysis showed that not only the cell length of the primary root but also root hair formation was affected by IPT treatment. To determine IPT’s influence on root hair formation, we analyzed the length and density of root hairs in the primary root formed during IPT treatment. In the differentiation zone of the wild-type root, root hair length was reduced by treatment with 12.5 µg/mL IPT but was reduced more extremely when treated with 75 µg/mL (Fig. 3A, B). The root hair density in the differentiation zone was not affected by treatment with 12.5 µg/mL IPT but was significantly reduced by treatment with 75 µg/mL IPT (Fig. 3B). Thus, the higher concentration of IPT has negative effects on both primary root growth and root hair formation. The lower concentration of IPT (12.5 µg/mL) has a negative effect on root hair elongation, whereas primary root elongation was enhanced by this concentration of IPT via JA and ET signal transduction. For a better understanding of the effect of 12.5 µg/mL IPT on root hair formation, the root hair length was analyzed in some phytohormone-related mutants. The reduction in root hair length by 12.5 µg/mL IPT treatment was also observed in jar1, ein2, salicylic acid (SA) biosynthesis-deficient mutant sid2, and a NahG transgenic plant expressing an SA-degrading enzyme, as well as in the wild-type (Fig. 3C, D), which indicated that JA, ET, and SA were not involved in the reduction in root hair length by IPT.
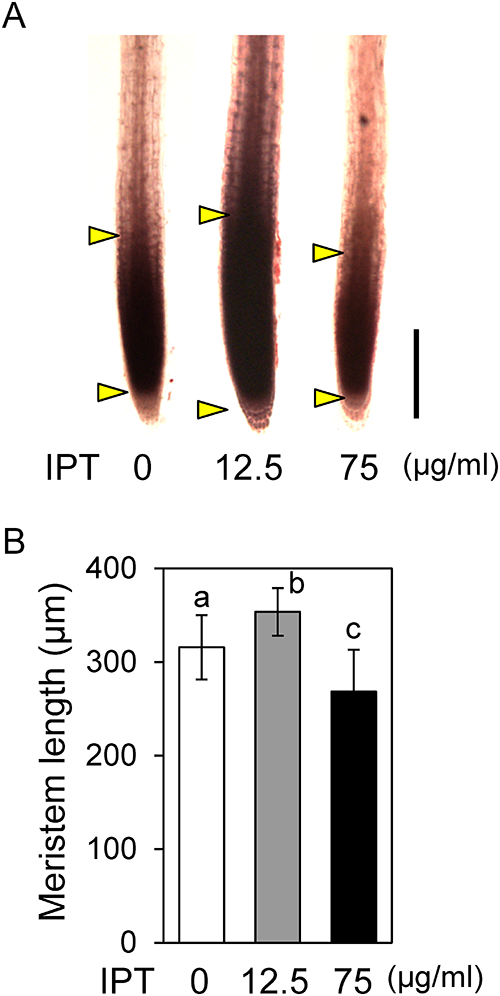
Treatment with 12.5 µg/mL IPT enhanced the primary root elongation, whereas the cell length in the differentiation zone was not affected. This fact suggested the possibility that the positive effect of IPT on primary root elongation is due to enhanced cell proliferation in the meristem. To examine this possibility, the root meristem sizes of IPT-treated seedlings were compared with those of non-treated control seedlings.15) Stereo microscopic analysis of acetocarmine-stained roots indicated that the meristem size of root treated with 12.5 µg/mL IPT was significantly larger than that of non-treated control (Fig. 4A, B). This result revealed that the meristem size was enlarged by treatment with 12.5 µg/mL IPT, probably due to increased cell proliferation in the meristem. In contrast, the meristem size was reduced by treatment with 75 µg/mL (Fig. 4A, B), indicating that cell proliferation in the meristem was suppressed by a higher concentration of IPT.
To understand the detailed mechanism of the increase in meristem size, the effect of 12.5 µg/mL IPT on the cell number in the meristem was examined via confocal microscopic analysis of the propidium iodide-stained root.16) The cell number of the wild-type root treated with 12.5 µg/mL IPT was ca. 17% higher than that of non-treated control root, indicating that IPT activated the cell division in the meristem (Fig. 5A, B). The increase in cell number by IPT treatment was not observed in the root of jar1 nor ein2 mutants, indicating that the activation of cell division by IPT treatment was dependent on JA and ET signals (Fig. 5B). Taken together with the cell length in the differentiation zone, this indicates that the lower concentration of IPT enhances primary root elongation by activating cell division in the meristem in a JA- and ET-dependent manner but not by enhancing the elongation process in elongation and differentiation zones.

On the other hand, the higher concentration of IPT (75 µg/mL) reduced the cell number compared to that of non-treated plants, which result was also observed in jar1 and ein2 mutants (Fig. 5A, B). Taken together, the negative effect of 75 µg/mL IPT on primary root elongation resulted from suppression of both cell elongation and cell division.
Our results demonstrate that the positive effect of 12.5 µg/mL IPT on primary root elongation in Arabidopsis is due to the JA- and ET-signal-dependent increased cell proliferation in the meristem but not due to changes of cell elongation. In contrast, a higher concentration of IPT (75 µg/mL) suppresses both cell division and cell elongation of primary root. In addition, the presented data demonstrate that IPT affects root hair formation, where root hair length was more sensitive to IPT than was its density. These data indicate that IPT (75 µg/mL) has negative effects on cell division, cell elongation, and root hair formation in primary root development, all of which are independent from JA and ET signals. In contrast, IPT’s positive effect on cell proliferation in the meristem is dependent on JA and ET.
In the cell division zone, IPT exhibited dual activities of promoting and suppressing cell proliferation, which should contribute to the enhancement and suppression of primary root elongation, respectively. Considering the necessity of JA and ET for IPT’s positive effect on cell proliferation, each of the dual activities is regulated independently of each other. On the other hand, for the cell elongation process from the end of the cell division zone to the differentiation zone via the elongation zone, IPT exhibited only the negative effect to suppress cell elongation, which should contribute to the suppression of primary root growth by a higher concentration of IPT. Whether these negative effects on cell proliferation and cell elongation are based on the same action of IPT is unknown, because no molecule and signal transduction in these activities have been identified.
Our previous study demonstrated that the intermediate concentration of IPT (37.5 µg/mL) had no effect on primary root growth in Arabidopsis, probably because the independently regulated positive and negative effects neutralized each other at this concentration.9) Based on the results presented here, at least three types of effects, increased and reduced cell division and suppression of cell elongation, should counterbalance one another. We have attempted to confirm the balanced effects histologically at this concentration, however, we have not yet succeeded because visible effects in the meristem and differentiation zone are faint under this condition.
Although JA and ET are known to suppress root growth, we previously reported, based on analyses using phytohormone-related mutants and inhibitors, that JA and ET take part in IPT’s positive effect on primary root elongation; this was confirmed as increased cell proliferation by histological analysis as shown here.9) Recently, it was reported that JA and ET promote cell division in the quiescent center of the root apical meristem; however, in those studies, these hormones function negatively in root elongation.17,18) Since cell division in the root growth process is intricately regulated by internal and external conditions in the root apical meristem that consists of many types of cells, its detailed regulation mechanisms remain to be clarified. Our finding offers an important clue to identifying the regulation mechanism of cell proliferation in the root apical meristem.
We thank Nihon Nohyaku Co., Ltd. for providing IPT. We also thank H. Takeuchi, A. Konishi, N. Kamikyo, and R. Komatsuzawa for technical assistances.