2020 年 45 巻 2 号 p. 86-94
2020 年 45 巻 2 号 p. 86-94
We investigated the dissipation of 27 pesticides in five Japanese soils at three temperatures and the variability of activation energies (Ea). The dissipation of total pesticides extracted sequentially using water and acetone was fitted to a single first-order (SFO) model. The Ea values calculated from the dissipation rate constants of the SFO model showed a normal distribution with a median of 61.1 kJ mol−1. The dissipation of water-extractable pesticides (i.e., phytoavailable pesticides) was fitted to a double first-order in parallel model with two dissipation rate constants: k1 and k2. The Ea values calculated from k1 and k2 showed normal or lognormal distribution, and the medians of the normal distribution calculated from k1 and k2 were 62.8 and 45.2 kJ mol−1, respectively. Furthermore, the method for estimating the biphasic dissipation of phytoavailable pesticides at different temperatures by using the median Ea values of the laboratory experiment was demonstrated in a field experiment.
Recently in Japan, some pesticides applied to previous crops and remaining in the soil have been detected in succeeding crops at levels exceeding the uniform residue limit of 0.01 mg kg−1 applied for all combinations of crops and pesticides without maximum residue limits.1,2) It is prohibited to sell or distribute crops that contain pesticides above the uniform residue limit. To prevent pesticide contamination of succeeding crops, understanding the dissipation behavior of phytoavailable pesticides in soil is crucial. Our previous research3) showed that the uptake concentrations of leafy vegetables cultivated in pesticide-applied soils had a higher positive correlation with the concentrations of water-extractable than total-extractable pesticides (i.e., the concentrations of pesticides extracted sequentially from soils using water and acetone). This result suggests that the phytoavailability of the residual pesticides in soil can be assessed using water-extractable concentrations in soil. In addition, we indicated that the dissipation behavior of water-extractable pesticides was quite different from that of total-extractable pesticides in soils with organic carbon (OC) content of 1% or more.4) In other words, the dissipation time for 50% (DT50) of water-extractable pesticides in soil was clearly shorter than that of total-extractable pesticides, and the dissipation of water-extractable pesticides was better fitted by biphasic models, i.e., a double first-order in parallel (DFOP) model as compared with a single first-order (SFO) model applied to the dissipation of total-extractable pesticides in soil.
The dissipation process in soil is one key factor governing the fate and behavior of pesticides in the environment and is affected by soil conditions such as temperature, moisture, pH and microbial activity.5,6) Japan is located in the mid-latitudes of the Northern Hemisphere, has a long landmass extending north and south and has four distinct seasons; thus, temperatures differ greatly depending on the region and season.7) Investigating the effect of temperature on the dissipation of pesticides in soils is very important for estimating the pesticide concentrations in succeeding crops taking temperature fluctuations into account.
The effects of temperature on the dissipation of pesticides expressed by the SFO model have been reported in many previous studies.8–15) In general, the relationship between the first-order dissipation rate constant (k) of the SFO model and temperature is analyzed using the activation energy (Ea) calculated from the Arrhenius equation.12) Using Ea values, it is possible to estimate the k values at different temperatures. The scientific report of the European Food Safety Authority (EFSA) indicated that the statistical distribution of median Ea values for each pesticide, derived from 99 datasets corresponding to 53 pesticides, was lognormal with a median value of 65.4 kJ mol−1.12) It is suggested that this value be used as a default in an environmental exposure assessment considering the effect of temperature. However, there is no report on the distribution of Ea values derived from the SFO model in Japanese soils including volcanic ash soil (Andisol). Although Andisols cover only about 0.8% of the earth’s surface,16) they play a crucial role in Japanese agriculture, considering that they cover approximately half of all upland fields in Japan.17)
In addition, it is necessary to examine the effect of temperature on the biphasic dissipation of water-extractable pesticides, so as to analyze the seasonal and regional difference in pesticide concentrations in succeeding crops. The DFOP model consists of two compartments: the first compartment with a faster dissipation rate governs the earlier part of the dissipation curve; and the second compartment with a slower dissipation rate governs the later part.18) The dissipation behavior in the first and second compartments follows first-order kinetics, and thus two dissipation rate constants are required: k1 and k2. So far, there is no report on the variability of each Ea value calculated for k1 and k2 according to the Arrhenius equation.
The aims of this study were to investigate the distribution of Ea values measured using typical Japanese soils, including Andisols, and 27 pesticides with different chemical classes and physico-chemical properties to determine whether the distribution of Ea values in Japanese soils differed from those reported by the EFSA, and to examine the differences in Ea values between k1 and k2 so as to estimate the biphasic dissipation of phytoavailable pesticides considering temperature fluctuations. Furthermore, the method used to estimate the first-order and biphasic dissipations of pesticides at different temperatures based on Ea values from laboratory experiments was verified by a field experiment.
The test conditions—chemicals, soils, laboratory incubation except for incubation temperature, sequential extraction of soils and pesticide analysis—are described in our previous report,4) in which soil samples were incubated at 25±2°C.
In brief, 27 pesticides (chemical purity of >97.0%) including 15 insecticides and 12 fungicides were selected on the basis of hydrophobicity, i.e., the range of their octanol–water partition coefficients (log Pow=−0.549–4.84) as shown in Table 1. On the basis of the analytical methods, pesticides were divided into three analytical groups: A, B and C. Five typical Japanese agricultural soils, with a wide range of OC contents (0.6–86.5 mg g−1), including two Andisols were used in laboratory experiments (Table 2).
| Compound | CAS no. | Pesticide typeb) | Substance group | log Powc) | log Swd) | Analytical groupe) | |
|---|---|---|---|---|---|---|---|
| Laboratory test | Field test | ||||||
| Dinotefuran | 165252-70-0 | I | neonicotinoid | −0.549 | 4.60 | A | A |
| Imidacloprid | 138261-41-3 | I | neonicotinoid | 0.570 | 2.79 | A | A |
| Dimethoate | 60-51-5 | I | organophosphate | 0.704 | 4.60 | A | |
| Clothianidin | 210880-92-5 | I | neonicotinoid | 0.905 | 2.53 | A | A |
| Thiacloprid | 111988-49-9 | I | neonicotinoid | 1.26 | 2.26 | A | A |
| Fosthiazate | 98886-44-3 | I | organophosphate | 1.68 | 3.95 | A | A |
| Metalaxyl | 57837-19-1 | F | phenylamide | 1.75 | 3.92 | A | A |
| Ethiprole | 181587-01-9 | I | phenylpyrazole | 1.99 | 0.964 | A | |
| Azoxystrobin | 131860-33-8 | F | methoxyacrylate | 2.50 | 0.826 | C | |
| Methidathion | 950-37-8 | I | organophosphate | 2.57 | 2.38 | A | |
| Fenobucarb | 3766-81-2 | I | carbamate | 2.78 | 2.62 | B | C |
| Boscalid | 188425-85-6 | F | pyridinecarboxamide | 2.96 | 0.663 | B | |
| Flutolanil | 66332-96-5 | F | phenylbenzamide | 3.17 | 0.904 | B | C |
| Procymidone | 32809-16-8 | F | dicarboximide | 3.30 | 0.391 | B | C |
| Fenitrothion | 122-14-5 | I | organophosphate | 3.32 | 1.28 | C | |
| Kresoxim-methyl | 143390-89-0 | F | oximinoacetate | 3.40 | 0.301 | B | |
| Tetraconazole | 112281-77-3 | F | triazole | 3.56 | 2.19 | C | |
| Chloroneb | 2675-77-6 | F | chlorophenyl | 3.58 | 0.903 | B | |
| Diazinon | 333-41-5 | I | organophosphate | 3.69 | 1.78 | C | |
| Propiconazole | 60207-90-1 | F | triazole | 3.72 | 2.18 | C | |
| Fipronil | 120068-37-3 | I | phenylpyrazole | 3.75 | 0.577 | C | |
| Cadusafos | 95465-99-9 | I | organophosphate | 3.85 | 2.39 | B | |
| Diclocymet | 139920-32-4 | F | carboxamide | 3.97 | 0.805 | C | |
| Trifloxystrobin | 141517-21-7 | F | oximinoacetate | 4.50 | −0.215 | B | |
| Tolclofos-methyl | 57018-04-9 | F | organophosphate | 4.56 | −0.150 | C | C |
| Tetradifon | 116-29-0 | I | bridged diphenyl | 4.61 | −1.11 | C | |
| Fenthion | 55-38-9 | I | organophosphate | 4.84 | 0.623 | B | |
a) Reprintcd with permission from ref.4. Copyright (2016) American Chemical Society.4) b) (I) Insecticides; (F) fungicides. c) Octanol–water partition coefficient and d) water solubility (mg/L) was obtained from the Pesticide Properties Database of IUPAC19) except for Pow of diclocymet and Sw of ethiprole, which was obtained from The Pesticide Handbook.20) e) Pesticides were divided into three analytical groups according to the analytical methods. The details of analytical methods are described in our previous report.21)
| Soil | Experiment | Classificationb) (USDA) | Classificationc) (Japan) | Textured) | OCe) (mg g−1) | CECf) (cmol(+) kg−1) | Clay (mg g−1) | pH (H2O) |
|---|---|---|---|---|---|---|---|---|
| LS1 | Laboratory | Entisol | Sand-dune Regosol | Sand | 0.6 | 3.4 | 24 | 7.5 |
| LS2 | Laboratory | Ultisol | Yellow soil | Light clay | 10.2 | 11.4 | 390 | 5.3 |
| LS3 | Laboratory | Entisol | Gray lowland soil | Silty clay | 14.6 | 18.2 | 253 | 5.8 |
| LS4 | Laboratory | Andisol | Andosol | Loam | 52.1 | 33.8 | 108 | 5.5 |
| LS5 | Laboratory | Andisol | Andosol | Silty loam | 86.5 | 35.4 | 18 | 5.8 |
| FS | Field | Andisol | Andosol | Silty loam | 49.3 | 26.0 | 73 | 6.3 |
a) Data are shown in our previous report.4) b) According to U.S. Department of Agriculture Soil Taxonomy.16) c) According to the criteria adopted by the Cultivated Soil Classification Committee.22) d) According to the International Society of Soil Science.23) e) Organic carbon content. f) Cation exchange capacity.
Air-dried soil with an 8 g of dry weight (DW) equivalent was placed in a 50 mL glass centrifuge tube. The water content of the soils was adjusted to 50–60% of the water-holding capacity during the test period. After a pre-incubation period of 10 days, 80 µL of an acetone stock solution (100 µg mL−1) of each pesticide group was added to a soil sample in a test tube; the initial pesticide concentration was 1 µg g−1 DW. Duplicate soil samples were incubated in darkness for 0, 2, 7, 14, 30, 60 and 120 days at 10±2°C and at 35±2°C. After each incubation period, the soil samples were analyzed using a sequential extraction method using distilled water and acetone.
Forty mL of distilled water was added to the soil samples in the tubes. The tubes were agitated on a thermostat shaker (TAITEC, Saitama, Japan) for 24 hr at 120 rpm and 25±2°C in darkness. After being shaken, mixtures were centrifuged at 1200×g for 30 min. Fifteen mL of the supernatant was withdrawn and used to quantify the water-extractable pesticides, and then 20 mL of supernatant was discarded. Subsequently, 30 mL of acetone was added to the remaining sample; the tubes were shaken in a thermostat shaker at 120 rpm and 25±2°C for 20 min, centrifuged at 1200×g for 10 min, and the supernatant was then carefully decanted. This extraction procedure was repeated twice more. The collected supernatant was evaporated in a rotary evaporator to reduce the volume to <15 mL, and then used to quantify the acetone-extractable pesticides in soil.
The aliquots (15 mL) of water extracts and concentrates (<15 mL) of acetone extracts from soils were analyzed using three different methods for each analytical group, as previously described in detail.21) The aliquots and concentrates were cleaned with a diatomite column (InertSep K-solute 20 mL; GL Sciences, Tokyo, Japan), and the following solid phase extraction cartridges were used: a PSA column (500 mg; Supelco, Bellefonte, PA, USA), an Accell CM column (500 mg; Waters, Milford, MA, USA) and an ENVI-Carb II/PSA column (500 mg/500 mg; Supelco) for analytical groups A, B and C, respectively. The cleaned samples were analyzed by liquid chromatography-tandem mass spectrometry for group A (Supplemental Tables S1 and S2) and gas chromatography-mass spectrometry for groups B and C (Supplemental Tables S3 and S4).
The concentration of water-extractable pesticides in soil, the CWE (µg g−1 DW), and the concentration of total-extractable (water- and acetone-extractable) pesticides in soil, the CTE (µg g−1 DW), were calculated as described in the previous study.4)
2. Data analysisTwo kinetic models, SFO (Eq. 1) and DFOP (Eq. 2), were used to describe the dissipation of the CWE and the CTE over time.18) The dissipation parameters were obtained using the least-squares method with Solver, the Microsoft Excel add-inn.
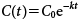 | (1) |
 | (2) |
where C(t) is the CWE or the CTE value after t days, C0 is the CWE or the CTE value at time t=0, k is the dissipation rate constant (day−1), f is the fraction of C0 applied to the first compartment, and k1 and k2 are the dissipation rate constants (day−1) in the first and second compartments, respectively. The goodness of fit for each model was assessed using the correlation coefficient (r) and model error (χ2 error, %), which was calculated as follows18):
 | (3) |
where C is the calculated value, O is the observed value, Ō is the mean of all observed values and χ2m,α is the tabulated chi-square value where m is the degrees of freedom—the number of measurements minus the number of model parameters—and α is the probability (5%).
For the SFO model, the DT50 (days), which is the time required for a 50% decrease in the CTE, was calculated as follows18):
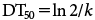 | (4) |
The relationship between the dissipation rate constant (k, day−1) and the temperature is represented by the Arrhenius equation12):
 | (5) |
where A is an empirical constant, Ea is the activation energy (kJ mol−1), R is the gas constant (8.3145 JK−1 mol−1) and T is the absolute temperature (K).
According to Eq. 5, the Ea values were calculated for k of the SFO model and for k1 and k2 of the DFOP model using laboratory incubation data at 10, 25 and 35°C. All data at 25°C used for data analysis were reported in our previous study.4) To ensure the quality of the Ea calculation, the following conditions for data selection had to be satisfied:
It is desirable that Arrhenius fits were conducted using k values for at least three temperatures. However, the number of Ea values calculated from three temperature points was small in this experiment, so the Ea values were also calculated using two temperature points.
3. Field experimentsTo verify the method for correcting the dissipation rate constants (k, k1 and k2) at different temperatures by using Ea values measured in laboratory experiments, field experiments were conducted in a field located in Tsukuba City, Japan, during spring–summer and autumn–winter periods. Details of the spring–summer experiment were described in a previous report,4) and the autumn–winter experiment was conducted in the same way as the spring–summer test except for crop cultivation. The pesticides (dinotefuran, imidacloprid, clothianidin, thiacloprid, fosthiazate, metalaxyl, fenobucarb, flutolanil, procymidone and tolclofos-methyl) and the characteristics of the field soil (FS) are shown in Tables 1 and 2, respectively. The spring–summer and autumn–winter experiments were started on May 12 and November 11, 2015, respectively, and the corresponding daily mean temperature of Tsukuba City during these periods ranged from 16–30°C (mean 23°C) and 1–17°C (mean 8°C), respectively. One liter of a mixed solution of the 10 pesticides (200 mg L−1 each), prepared by dilution of commercial formulations (emulsifiable concentrates, water-soluble powders and water-dispersible powders) with water, was evenly applied to triplicate plots (1 m×1 m) using a watering can. Subsequently, the soil surface (approximately 0–20 cm depth) was tilled with a walking-type tilling apparatus. Furthermore, the seeds of komatsuna (Brassica rapa var. perviridis ‘Yokattana’) were sown and cultivated in each plot only for the autumn–winter experiment. Four cores were taken from each plot at soil depth of 0–10 cm at 0 (immediately after tilling), 2, 7, 14, 21, 28, 35, 42, 49, 56, 63, 70, 77 and 84 days and at 0, 28 and 64 days after the application of pesticides for the spring–summer and autumn–winter experiments, respectively. The cores for each plot were combined and mixed well. The sampled soil equivalent to 5 g DW was analyzed using a sequential extraction method in the same way as in the laboratory experiment.
The temperature correction of the dissipation rate constants using Ea values from laboratory experiments was performed using the following equation12):
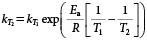 | (6) |
where kT1 and kT2 are the dissipation rate constants at temperatures T1 (23°C, i.e., 296 K of the mean temperature during the spring–summer experiment) and T2 (8°C, i.e., 281 K of the mean temperature during the autumn–winter experiment). The kT1 values were calculated from the SFO (Eq. 1) and DFOP (Eq. 2) models using the dissipation data of the spring–summer experiment, and then the dissipation of pesticides in the autumn–winter experiment was estimated using kT2 calculated by Eq. 6. We compared the estimated values with the measured values on 28 and 63 days after the application of pesticides for the autumn–winter experiment.
4. Statistical analysisThe statistical outliers of the calculated Ea values were found with the Smirnov–Grubbs test at the 5% level of significance using Microsoft Excel 2010, and then the normality or lognormality (log indicates the natural logarithm) of the Ea distribution was checked by Shapiro–Wilk’s test using SPSS Statistics 25 (IBM Corporation, Armonk, NY, USA). Normality or lognormality assumptions were rejected if the p-value for fit was below 0.05. A compound-specific Ea value was reported to exist; for example, the Ea values of phenylureas were statistically lower than those of non-phenylureas.12) Therefore, considering inter-compound variability, the tests for normality or lognormality of Ea distribution were performed not only with individual Ea values across pesticides (all data) but also with the medians of Ea values among test soils (n≥2) for each pesticide.12)
The details of fitting the SFO and DFOP models to the dissipation data for all pesticides and all soils are summarized in Supplemental Tables S5 and S6. The dissipation of pesticides in soils showed different behaviors depending on the temperature and combinations of pesticides and soils, and there were differences between total-extractable and water-extractable concentrations (CTE and CWE). Figure 1 shows an example of the fungicide propiconazole in soils LS1 and LS5. The dissipation in soils was influenced by temperature, and the dissipation rate constants in both soils increased with increasing temperature.

Overall, the dissipation of the CTE was basically fitted by the SFO model as shown in our previous study.4) The χ2 error value of the SFO model was mostly below 15% (Fig. 2), which is the threshold value suggested by the Forum for the Co-ordination of Pesticide Fate Models and their Use.18) However, focusing on the dissipation of the CTE at a high temperature (35°C), the χ2 error values of the SFO model tended to be higher than those of the DFOP model (Fig. 2). In such cases, it was preferable to use the DFOP model to describe the dissipation of the CTE.

The dissipation behavior of the CWE in soil LS1 was similar to that of the CTE (Fig. 1). Among the tested soils, LS1, which had extremely low OC content, showed the lowest apparent sorption coefficient (Kd,app)4) and the highest water extractability (CWE divided by CTE). That is, most of the pesticide residues in LS1 were extracted by water. Overall, the χ2 error values of the SFO model in LS1 for the CTE and the CWE were almost the same as those of the DFOP model (Fig. 2); thus, the dissipation of pesticides in LS1 could be expressed by the SFO model.
However, the dissipation of the CWE in soil LS5, which had the highest OC content, was faster than that of the CTE (Fig. 1). As previously reported,4) the Kd,app values of pesticides in LS5 increased with time, i.e., the water extractability of pesticides decreased with time, so that differences in the dissipation rate constants of the CWE and the CTE arose. The dissipation of the CWE in LS5 was not fitted by the SFO but by the DFOP model regardless of temperature (Fig. 1). For the dissipation of the CWE at 35°C, the χ2 error value of the SFO model was 29.9%, and the visual fit was not acceptable (Fig. 1). In this case, the DFOP model with a χ2 error value of 6.4% was preferable for expressing the dissipation of the CWE (Fig. 1). A good fitting of the DFOP model to the dissipation data of the CWE and differences in the χ2 errors of the SFO and DFOP models were also observed in soils LS2, LS3 and LS4, and the difference in the χ2 error between the SFO and DFOP models tended to increase in the two Andisols (LS4 and LS5) with higher OC content (Fig. 2). The reason for this was that the dissipation of the CWE was strongly affected by a time-dependent increase in Kd,app,4) the rate of which was high for soil with high OC content and showed a biphasic pattern, as shown in our previous study.4) Thus, the dissipation of the CWE in soils, except for LS1 (OC=0.6 mg g−1), should be described by the DFOP model regardless of the temperature.
2. Distribution of Ea values derived from the dissipation of total-extractable pesticides in laboratory experimentsIn order to investigate the effect of temperature on the dissipation of the CTE, the Ea values were derived from Eq. 5. The statistical distributions of the Ea values calculated using the SFO and DFOP models are shown in Supplemental Table S7. The Ea values for the DFOP model of soil LS1 were not obtained because the dissipation of both the CTE and the CWE in LS1 could be expressed by the SFO model as described in Section 1 above. The number of individual Ea values calculated for k1 and k2 of the DFOP model was low compared to that of the SFO model (Supplemental Table S7). This was because the dissipation of the CTE for some combinations of pesticides and soils tended to be fitted not by the DFOP model but by the SFO model (in some cases, the f values of DFOP models for the CTE were zero or one, Supplemental Table S5), the Arrhenius fits were not good, and the calculated Ea values were negative in many cases. Furthermore, the k2 values of the DFOP model at low temperatures were often calculated as zero (Supplemental Table S1); thus, the calculated number of Ea values for k2 was particularly low. When the k2 values are zero, it means that the pesticide is not dissipated in the second soil compartment. However, further experiments with a longer incubation period are needed to demonstrate that k2 values really are zero.
For the SFO model, the individual calculated Ea values using the k values at three temperatures were statistically described by a normal distribution, that is, the p-values of the Shapiro–Wilk’s test exceeded 0.05, and the median Ea value was 62.9 kJ mol−1 (Supplemental Table S2). Additionally, the median Ea values of each pesticide were normally distributed and the median value of the distribution was 61.1 kJ mol−1 (Table 3). Thus, the median Ea of the distribution derived from the median Ea values of each pesticide was almost the same as that derived from individual Ea values. Focusing on the differences in Ea values among soils (Table 4), the average Ea value of soil LS4, an Andisol, was statistically equivalent to those of other soils. Likewise, the average Ea value for soil LS5, also an Andisol, did not significantly differ from that of other soils. Therefore, no specificity of Ea distribution was observed for Andisols, which are the major upland soils in Japan. The EFSA reported that the distribution of median Ea values of the SFO model for each pesticide measured mainly using European soils had a median value of 65.4 kJ mol−1 and this value would be available as a default value for the environmental exposure assessment.12) The median value reported by the EFSA was nearly the same as the corresponding value in our study—of 61.1 kJ mol−1—measured using Japanese soils. These results imply that a median Ea value can be used for environmental exposure assessments in Japanese soils when considering the effects of temperature. However, the Ea values varied depending on the combinations of pesticides and soils. Therefore, if soil- and compound-specific Ea values have been measured, the measured values should be used to assess environmental exposure.
| Kinetic model | Dissipation rate constant | Distribution check | ||||||
|---|---|---|---|---|---|---|---|---|
| Distribution | N | Ea (kJ mol−1)h) | Shapiro–Wilk’s test (p) | |||||
| Avg. | Stdi) | Median | ||||||
| TEa) | SFOc) | k | Normality | 23 | 58.5 | 24.4 | 61.1 | 0.231 |
| Lognormality | 23 | 50.4 | 0.56 | 61.1 | 0.001 | |||
| DFOPd) | k1e) | Normality | 20g) | 104.9 | 58.5 | 97.4 | 0.124 | |
| Lognormality | 22 | 86.5 | 0.74 | 94.2 | 0.479 | |||
| k2f) | Normality | 15g) | 48.5 | 17.7 | 44.4 | 0.091 | ||
| Lognormality | 16 | 48.3 | 0.45 | 45.0 | 0.599 | |||
| WEb) | DFOPd) | k1e) | Normality | 26 | 64.2 | 17.2 | 62.8 | 0.434 |
| Lognormality | 24g) | 63.6 | 0.26 | 63.2 | 0.079 | |||
| k2f) | Normality | 18g) | 45.1 | 12.3 | 45.2 | 0.560 | ||
| Lognormality | 21 | 48.0 | 0.48 | 48.3 | 0.449 | |||
a) Total-extractable pesticides in soil. b) Water-extractable pesticides in soil. c) Single first-order model. d) Double first-order in parallel model. e) Dissipation rate constant of the first compartment. f) Dissipation rate constant of the second compartment. g) Number of data points except statistical outliers found with Smirnov–Grubbs test at the 5% level of significance. h) Ea values were calculated from two data points or more. i) Standard deviation. In case of lognormality, the values mean Std (ln Ea).
| Soils | TRa) | WEb) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SFOc) | DFOPd) | DFOPd) | |||||||||||||
| k | k1e) | k2f) | k1e) | k2f) | |||||||||||
| N | Avg.g) | Median | N | Avg.g) | Median | N | Avg.g) | Median | N | Avg.g) | Median | N | Avg.g) | Median | |
| LS1 | 16 | 62.8 A | 68.3 | NDh) | NDh) | NDh) | NDh) | ||||||||
| LS2 | 26 | 61.5 A | 62.7 | 17 | 113.6 A | 101.3 | 18 | 44.5 A | 47.8 | 20 | 93.8 B | 91.6 | 16 | 81.9 A | 53.0 |
| LS3 | 20 | 62.4 A | 61.3 | 15 | 151.9 A | 158.5 | 7 | 51.9 A | 43.8 | 24 | 52.9 A | 51.9 | 18 | 41.4 A | 42.9 |
| LS4 | 18 | 35.2 A | 28.5 | 11 | 112.3 A | 53.7 | 11 | 56.0 A | 46.3 | 25 | 72.7 AB | 68.8 | 24 | 57.3 A | 46.7 |
| LS5 | 18 | 55.4 A | 51.3 | 14 | 109.2 A | 71.6 | 10 | 75.7 A | 54.9 | 17 | 68.0 A | 62.0 | 17 | 72.3 A | 71.3 |
a) Total-extractable pesticides in soil. b) Water-extractable pesticides in soil. c) Single first-order model. d) Double first-order in parallel model. e) Dissipation rate of the first compartment. f) Dissipation rate of the second compartment. g) Values with the same letter are not significantly different at p<0.05, according to ANOVA followed by Tukey’s multiple comparison test. h) No data.
For the Ea values derived from the DFOP model, the individual Ea values calculated from the k1 and k2 values at two or more temperatures were statistically described by a lognormal distribution if statistical outliers were excluded from the data set, and the median Ea values of the distributions for k1 and k2 were 99.7 and 46.8 kJ mol−1, respectively (Supplemental Table S7). Additionally, the distributions of the median Ea values of each pesticide were also lognormal and the median Ea values of the distributions for k1 and k2 were 94.2 and 45.0 kJ mol−1, respectively (Table 3).
3. Distribution of Ea values derived from the dissipation of water-extractable pesticides in laboratory experimentsThe statistical distributions of Ea values calculated from k1 and k2 of the DFOP model for the CWE were investigated in the same way as for the CTE (Table 3 and Supplemental Table S7). The number of individual Ea values calculated from k1 and k2 for the CWE was larger than that for the CTE (Supplemental Table S7). This is likely because the dissipation of the CWE was better fitted by the DFOP model than was the dissipation of the CTE. As described in Section 2 above, the dissipation of the CTE tended to be fitted not by the DFOP model but by the SFO model. Therefore, the number of Ea values calculated using the DFOP model for the CTE was smaller than that for the CWE.
On the whole, the distributions of Ea values except for statistical outliers were normal or lognormal, and the median Ea values of the distributions were very similar regardless of the methods of calculating Ea (Table 3 and Supplemental Table S7). The normal distributions, whose p-values for Shapiro–Wilk’s test were higher than those of lognormal distributions, of the median Ea values of each pesticide for k1 and k2 had median values of 62.8 and 45.2 kJ mol−1, respectively (Table 3).
Although differences in the median Ea values for k2 of the CTE and the CWE were not observed, the median Ea value for k1 of the CTE was greater than that of the CWE (Table 3). One reason for this was that the dissipation of the CWE could be described by the DFOP model regardless of temperature, whereas the DFOP model provided a better fit for the dissipation data of the CTE at a high rather than a low temperature (Figs. 1 and 2 ). That is to say, the slope on the earlier part (the first compartment) of the dissipation curve of the CTE at a high temperature was considerably greater than that at a low temperature; thus, the slope of Arrhenius fits (the Ea value) was large. Actually, the ratio of k1 at 35°C to that at 10°C for the CTE was higher than that for the CWE (Fig. 1). In addition, the median Ea values for k2 were lower than those for k1 for both the CTE and the CWE (Table 3). This result implies that the effect of temperature on the dissipation of pesticide in the second compartment with a slower dissipation rate was small. In other words, the pesticides in the second compartment, which sorb to soil particles and have low bioavailability and biodegradability, were insensitive to temperature as compared with those in the first compartment.
There were no remarkable differences in the Ea values for both k1 and k2 among test soils for the CWE, and the specificity of the Ea distribution for Andisols was not observed in the same way as of the Ea values derived from k of the SFO model for the CTE (Table 4). Focusing on the difference in the Ea values among test pesticides, the median Ea values of each pesticide for both k1 and k2 exhibited high variability. However, the reason for this could not be explained by the differences in the chemical classes and physico-chemical properties of pesticides (Supplemental Table S8).
4. Field experimentsThe dissipation of pesticides during the autumn–winter period was estimated using the measured concentration on day 0 of the autumn–winter experiment, the dissipation rate constants for spring–summer experiment, and the Ea values from the laboratory experiment. The median of the distribution, which had a relatively high Shapiro–Wilk’s test p-value, of the median Ea values for each pesticide was used for the estimation using Eq. 6. The corresponding medians for k, k1 and k2 of the CTE were 61.1, 94.2 and 45.0 kJ mol−1, respectively; those for the k1 and k2 of the CWE were 62.8 and 45.2 kJ mol−1, respectively (Table 3).
When the dissipation of pesticides was estimated using the DFOP model, not only k1 and k2 values but also f values were also necessary. Therefore, the variability of f values at three temperatures was investigated. Overall, although the f values tended to increase with increasing temperature (Supplemental Tables S5 and S6), the geometric means of the coefficients of variation calculated from the f values at three temperatures for the CTE and the CWE were 40.0 and 26.0%, respectively; thus, f values exhibited relatively low variability. The variability of f values differed depending on the combinations of pesticides and soils. However, the variability of f values at three temperatures could not be explained by the difference in physical and chemical properties of pesticides or soils. Therefore, temperature correction of f values was not conducted for estimating the dissipation of pesticides by the DFOP model.
The comparison between the measured and estimated values for nine pesticides, except fenobucarb, whose measured values were below the limit of quantification, in the autumn–winter field experiment, is shown in Fig. 3. When temperature correction of the dissipation rate constants was not performed (Case 1), the estimated values tended to underestimate the measured values. The ratios of the measured values to the values estimated by the SFO model for the CTE ranged from 0.7–7.4. In particular, the measured values of thiacloprid, fosthiazate, procymidone and tolclofos-methyl, which had relatively low DT50 values (Supplemental Table S9), exceeded the estimated values by more than threefold. However, values estimated using temperature-corrected dissipation rate constants according to Eq. 6 (Case 2) were roughly the same as the measured values (Fig. 3), and the differences between measured and estimated values were smaller than those for Case 1. The ratios of the measured values to the values estimated using the SFO models for the CTE ranged from 0.4–1.0.

The estimation accuracy of the SFO model for the CTE was almost the same as that of the DFOP model for both the CTE and the CWE (Fig. 3). The ratios of the measured values to the values estimated using the DFOP models for the CTE and the CWE in Case 1 ranged from 0.7–4.4 and 0.7–10.4, respectively, whereas the corresponding ratios in Case 2 were 0.4–1.0 and 0.4–1.2. Thus, the estimated values in Case 2 showed good agreement with the measured values. This result suggests that temperature-dependent biphasic dissipation can be estimated using each different Ea value corresponding to k1 and k2.
In conclusion, the median of the distribution of Ea values for first-order dissipation derived from Japanese soils including Andisols was almost the same as that derived from European soils as reported by the EFSA.12) The variability of Ea values for k1 and k2 calculated from the biphasic DFOP model could also be expressed by normal or lognormal distributions, and the median Ea of the distribution for k1 was higher than that for k2. Furthermore, the biphasic dissipation of water-extractable pesticides in a field soil, which was important for evaluating pesticide residue in succeeding crops, could be estimated considering temperature fluctuations, i.e., seasonal differences, by using each median Ea value corresponding to k1 and k2. Further verification experiments using other pesticides and fields are required to establish such an estimation method for biphasic dissipation.
We thank Shintaro Kanbayashi (Waseda University, Tokyo, Japan) for his help with the laboratory experiments. The test soils were kindly supplied by Aichi Agricultural Research Center and Tochigi Prefectural Agricultural Experiment Station. This research was supported by the Environmental Research and Technology Development Fund (5-1302) of the Ministry of the Environment, Japan.
The online version of this article contains supplementary materials (Supplemental Tables S1–S9), which are available at http://www.jstage.jst.go.jp/browse/jpestics/.