2020 年 45 巻 4 号 p. 206-215
2020 年 45 巻 4 号 p. 206-215
Flutianil, chemically characterized as a cyano-methylene thiazolidine, showed antifungal activity against powdery mildew on various crops but not any other plant pathogens tested. Flutianil showed high residual and translaminar activities and rainfastness against Podosphaera xanthii. It also exhibited curative activity against P. xanthii on cucumber at an extremely low concentration of 10 mg/L. There was no cross-resistance between flutianil and other existing fungicides. Morphological studies revealed that flutianil did not inhibit the early infection behavior of Blumeria graminis f. sp. hordei i.e., conidium bursting, primary and appressorial germinations, appressorium development and hook formations, but it did inhibit haustorium formation and further fungal development. Nutrient absorption by haustoria and subsequent secondary hyphal elongation was inhibited by flutianil but not by the fungicide that showed a similar inhibitory pattern up to the haustorium formation stage of the infection process. These findings suggest that flutianil might have a novel mode of action.
Powdery mildew is a widespread plant disease caused by a variety of fungal species of Erysiphales.1) Powdery mildew pathogens are known as obligate biotrophs that depend on living plant cells for their survival and reproduction.2) It is one of the most destructive fungal diseases in many crops and often causes major economic losses in agricultural production.3) For the control of powdery mildew, various classes of chemical fungicides—such as benzimidazoles, morpholines, 2-aminopyrimidines, triazoles (sterol demethylation inhibitors: DMIs), strobilurins (QoI), azanaphthalenes, and benzophenones—have been used. The fungicides belonging to each of these classes have a specific action target in the pathogen and high efficacy with low-concentration treatment.3–8) Frequent use of these selective fungicides has resulted in the emergence of resistant populations of the target pathogen.9) In particular, powdery mildew pathogens were shown to have a high potential for developing resistance to single site-targeted fungicides, the overexpression of the site-targeted gene, and/or efflux transportation that pumps out endogenous compounds from the cells. Therefore, demand for a fungicide with a novel mode of action against powdery mildew has been increasing.
This paper deals with the biological properties of a novel fungicide, flutianil, (Gatten®, (2Z)-{[2-fluoro-5-(trifluoromethyl)phenyl]sulfanyl}[3-(2-methoxyphenyl)-1,3-thiazolidin-2-ylidene]acetonitrile) (chemical structure shown in Fig. 1), which was discovered through extensive studies on the synthesis and biological screening of the cyano-methylene thiazolidine chemical class.10) Flutianil has proved to be a unique fungicide, since its antifungal spectrum covers only powdery mildew species, including Podosphaera xanthii, Sphaerotheca fuliginea, Erysiphe necator, Sphaerotheca aphanis var. aphanis, Blumeria graminis f. sp. tritici (Bgt), and Blumeria graminis f. sp. hordei (Bgh). We will report on flutianil’s antifungal spectrum, residual and translaminar activities, rainfastness, cross-resistance with other existing fungicides, and curative activity, as well as its inhibitory effect on haustorium formation and sporulation.

Since flutianil is currently classified as a part of Fungicide Resistance Action Committee (FRAC) Group U 13,11) meaning it has an unknown mode of action, a description of the findings that led to the elucidation of the mode of action of flutianil is also made.
Synthesized flutianil and cyflufenamid analytical standards were used for all laboratory studies, including in vitro fungal spectrum and microscopy analyses. Triflumizole, azoxystrobin, tolfenpyrad, quinoxyfen, benomyl, and chinomethionat were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Metrafenone was purchased from Sigma-Aldrich (Tokyo, Japan). Stock fungicide solutions were prepared by dissolving each compound in acetone and diluting it with distilled water to make a test solution with <1% acetone (v/v).
For pot tests and the cross-resistance study, the following formulated ingredients were used: 50 g/kg flutianil EC, 200 g/kg azoxystrobin SC, 300 g/kg triflumizole WP, a mixture of 34 g/kg cyflufenamid and 150 g/kg triflumizole WDG, 200 g/kg penthiopyrad SC, 250 g/kg chinomethionat WP, 300 g/kg iminoctadine albesilate SC, 250 g/kg quinoxyfen SC, and 250 g/kg tebuconazole EW.
2. Biological properties2.1. Preparation of test plantsWheat seeds (Triticum aestivum cv. Chikugoizumi) were sown in plastic trays with 128 cells (59.5×30×50 mm) filled with culture soil (Aisai 1-go, Katakura & Co-Op Agri, Tokyo, Japan). The plastic trays were placed in a growth chamber at 23°C with 60% relative humidity (RH) under a 16 hr photoperiod for 7 days. Seeds of cucumber (Cucumis sativus cv. Suzunari-suyo), eggplant (Solanum melongena cv. Senryo), pea (Pisum sativum cv. Narikoma-sanjyunichi), and tomato (Solanum lycopersicum cv. Momotaro Eight) were sown in 7.5 cm diameter polyethylene pots filled with the same culture soil used for wheat sowing and placed in a greenhouse for 21 days. Grapes (Vitis vinifera cv. Pione) were planted in 9 cm diameter polyethylene pots filled with the same culture soil used for sowing wheat and placed in a greenhouse. Rice seeds (Oryza sativa subsp. japonica cv. Koshihikari) were sown in plastic trays with 128 cells (59.5×30×50 mm) filled with culture soil (Ube-ryujyobaido, Katakura & Co-Op Agri, Tokyo, Japan) and placed in a greenhouse. Rice plants grown to approximately the 1.2 leaf stage (about two weeks after seeding) were used for experiments. Strawberry plants (Fragaria×ananassa cv. Akihime) were planted in 7.5 cm diameter polypropylene pots filled with the same culture soil used for sowing wheat and grown in a greenhouse.
2.2. Fungicide treatment and pathogen inoculationA conidial suspension (105 conidia/mL) of P. xanthii or the conidial dust of S. fuliginea, which had been maintained on cucumbers or eggplants in a laboratory, respectively, was inoculated onto the leaves 3 hr after treatment with 10 mg/L of flutianil (unless otherwise noted, the treatment concentration of flutianil was 10 mg/L). For E. necator, a conidial suspension (105 conidial/mL) was inoculated onto grape leaves 3 hr after treatment with flutianil. In cases of Bgt and Bgh, wheat or barley was treated with flutianil and inoculated with powdery mildew by exposing each treated plant to a conidial dust of Bgt and Bgh strains that had been maintained on each plant in a laboratory. In the case of B. cinerea, flutianil treatment and the inoculation procedure are as follows: flutianil-treated cucumber cotyledons were excised and placed in a wet paper-lined plastic case to maintain the humidity in the case. A 50 µL spore suspension of B. cinerea (1×105 spores/mL) in YG medium (10 g yeast extracts and 20 g glucose per liter) was placed onto the cucumber leaves and covered with a paper disc (Toyo Roshi Kaisha, Tokyo, Japan). A 50 µL YG medium was then added onto the paper disc. For P. fulva and M. nattrassii, a conidial suspension (106 conidia/mL) prepared from a potato dextrose agar (PDA) culture was inoculated onto tomato or eggplant leaves 3 hr after treatment with flutianil. The inoculated plants were then kept in a dew chamber (100% RH) at 25°C for 24 hr and then kept in a growth chamber. In the case of G. cingulata, flutianil treatment and the inoculation procedure are as follows: G. cingulata was grown on a PDA medium plate for 5 days in darkness at 25°C. A potato sucrose (PS) liquid medium containing 20% (v/v) potato broth and 20 g/L sucrose was inoculated with the tip of a mycelium disc and then shaken at 130 rpm for 5 days at 25°C to produce conidia. The conidial suspension (1.0×106 conidia/mL) thus obtained was inoculated to flutianil-treated strawberry plants. The inoculated plants were then kept in a dew chamber (100% RH) at 25°C in the dark for 24 hr and then in a growth chamber. For P. infestans the conidial suspension (105 conidia/mL) which was grown on detached tomato leaves at 15°C for 7 days, was inoculated onto the flutianil-treated plants. The inoculated plants were kept in a dew chamber (100% RH) at 20°C for 24 hr and then in a growth chamber. For P. oryzae inoculation, P. oryzae was isolated from the field and grown in a laboratory on a culture medium containing 5% (w/v) oatmeal and 2% (w/v) sucrose for 14 days at 25°C in darkness. Spore formation was induced by placing the cultures under a 20 W blacklight blue (BLB) fluorescent lamp for 3 days at 25°C. The spore suspension (105 conidia/mL) thus obtained was sprayed onto rice leaves 3 hr after treatment with flutianil. The inoculated plants were kept in a dew chamber (100% RH) at 25°C in darkness for 24 hr and then in a growth chamber.
2.3. Evaluation of activityThe lesions on the inoculated leaves were observed, and the grading ranged from 0 to 4, where 0 indicated no lesions, 1 indicated lesions occupying less than 5% of the leaf area, 2 indicated lesions occupying 5–25% of the leaf area, 3 indicated lesions occupying 25–50% of the leaf area, and 4 indicated lesions occupying more than 50% of the leaf area. A control value (CV) was calculated using the following formula:
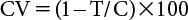 |
Here, T represents the index of the leaf area covered with lesions on the treated plants, and C represents the index of the leaf area covered with lesions on the untreated plants.
3. Residual activity and rainfastnessTo examine the residual activity of flutianil, flutianil-sprayed cucumber seedlings were kept in a greenhouse for 14 days, followed by inoculation with P. xanthii, and the antifungal activity was measured. To examine the rainfastness, flutianil-sprayed cucumber plants were kept for 24 hr in a greenhouse and then exposed to artificial rain (40 mm/hr; raindrop particle size=1.75 mm) for 2 hr using an artificial rain generator (DIK-6000-S, Daiki Rika Kogyo, Saitama, Japan). The plants were then inoculated with a conidial suspension of P. xanthii and subjected to activity measurement.
4. Translaminar and systemic activities of flutianil in cucumber plantsTranslaminar and systemic activities of flutianil were investigated in a greenhouse. To examine the translaminar activity, a sufficient amount of flutianil was applied with a brush either to the adaxial (a) or the abaxial (b) surfaces of cucumber leaves; both sides of the leaves were inoculated with P. xanthii after 24 hr and the control effect was measured. To examine systemic activity, a sufficient amount of flutianil solution was applied with a brush to the base or apex of cucumber leaves and with a pipette to the soil around the cucumber plants. The plants were inoculated with P. xanthii 24 hr after flutianil treatment, and the control effect was measured.
5. Cross-resistance and sensitivity study of flutianil5.1. Cross-resistance study with P. xanthii and S. aphanis var. aphanisThe sensitivities of flutianil to the powdery mildew isolates (P. xanthii) collected from cucumbers in 12 locations in Japan and to the powdery mildew isolates (S. aphanis var. aphanis) collected from strawberries in 8 locations in Japan were compared with those to other fungicides of various chemical classes, i.e., azoxystrobin (QoI), triflumizole (DMI), a mixture of cyflufenamid and triflumizole, penthiopyrad, chinomethionat, and iminoctadine albesilate. The isolates mentioned above were collected from 2007 to 2013 in cucumber or strawberry cultivation houses. The efficacies of flutianil and other fungicides on the P. xanthii isolates were determined by pot test. The efficacies of flutianil and other fungicides on S. aphanis var. aphanis were determined by using leaf discs of strawberry plants (cv. Nyohou). Young strawberry leaves were used for the experiment because of their high sensitivity to pathogens. The leaves were then cut into discs (10 mm in diameter) which were placed into Petri dishes with their upper surfaces down. The concentration of flutianil used for treatment was 0.03 to 10 mg/L. The concentration of the other fungicides was set as stated on the product labels. The test solutions were then sprayed onto the strawberry leaf discs. After air-drying, the leaf discs were inoculated, and the discs were kept in a greenhouse for 7 days. Then, each disc was visually graded on the degree of fungus attack by observing the entire disc surface using a stereoscopic microscope (Nikon, Tokyo, Japan). The grading ranged from 0 to 4, where 0 indicated no lesions, 1 indicated lesions occupying less than 5% of the disc area, 2 indicated lesions occupying 5–25% of the disc area, 3 indicated lesions occupying 25–50% of the disc area, and 4 indicated lesions occupying more than 50% of the disc area. Fungicide efficacy was calculated using the method described in Section 2.3.
5.2. Cross-resistance study with E. necator isolates in EuropeTen E. necator isolates were selected from a 30 E. necator-isolate library from Europe and maintained in our laboratory. These isolates have different sensitivities to sterol demethylation inhibitor (DMI) fungicides and/or quinoxyfen. Cross-resistance tests were carried out using leaf discs of vine plants (Vitis vinifera cv. Cinsaut). Young glossy leaves were collected because of their high sensitivity to the pathogen. Vine leaves were sterilized for 30 sec in a 50 g/L sodium hypochlorite solution and then rinsed twice with sterile water before being dried by sandwiching them between two pieces of sterile filter paper. The leaves were then cut into discs (18 mm in diameter) and placed in petri dishes followed by treatment with fungicides. The treatment concentrations were 0.003, 0.01, 0.33, 0.1, 0.3, 1, and 3 mg/L for flutianil; 0.01, 0.1, 0.3, 1, 3, 10, 30, and 100 mg/L for tebuconazole; and 0.1, 0.3, 1, 3, 10, 30, and 100 mg/L for quinoxyfen. The fungicides were applied directly to the upper surfaces of the leaf discs.
6. Curative treatment with flutianilFlutianil (10 mg/L) was applied to the cucumber leaves, which were inoculated with a conidial suspension 1 day after the inoculation (pre-symptoms), and then 5, 7, 9, 12, and 14 days after the inoculation (post-symptoms). The curative activity of flutianil against P. xanthii was evaluated by visual assessment each day up to 14 days after inoculation. Mycelium formation and sporulation were observed 7 days after the application. Observations were made using a light microscope (Keyence VHX-200, Osaka, Japan) on four sites of the first cucumber leaves, and the experiments were repeated three times. A secondary infection study was conducted using the cucumber plants from the curative activity study as an infection source. A non-infected cucumber cotyledon was inoculated by rubbing it against the infected plant. Seven days after inoculation, the presence or absence of powdery mildew in cucumber cotyledons was observed.
7. Light microscopic observation of infection behaviorBarley powdery mildew (Bgh) was used as the observation target of the infection behavior. Barley seedlings (Hordeum vulgare cv. Kobinkatagi) were grown in culture soil in a growth chamber under fluorescent light (approx. 11.8 W/m) with a 12 hr photoperiod at 20°C and 70% RH. Coleoptiles were excised from barley seedlings 7 days after sowing and single-cell epidermal layers of partially dissected coleoptiles were prepared as previously described.12) The coleoptiles floated on 1 mM calcium chloride (CaCl2) in a Petri dish containing 10 mg/L of flutianil and other existing fungicides were inoculated with freshly harvested Bgh conidia using a brush and incubated at 20°C for 24 hr. Each coleoptile was placed in 1 mM CaCl2 on a glass slide. Infection behaviors—conidium bursting, primary germ tube (PGT) germination, appressorial germ tube (AGT) germination, appressorial (APP) formation, APP hook formation, and haustorium formation of Bgh conidia on coleoptiles—were observed under a light microscope (Nikon, Osaka, Japan) at 200×magnification.
In order to measure the effect of the addition of nutrients to haustoria for the elongation of secondary hyphae, the coleoptiles in the Petri dish were incubated with the test compounds at 20°C for 24 hr, followed by addition of 0.1 M glucose as a nutrient. The coleoptiles were placed on a slide glass in preparation for microscopic observation. Then, 24 hr later, the elongation of secondary hyphae was measured using eyepiece scales.
8. Scanning electron microscopyCucumber plants at the 1.2-leaf stage were inoculated with P. xanthii and incubated for 7 days in a greenhouse. Flutianil (10 mg/L) was applied to the inoculated cucumber leaves and then maintained for 7 days in a greenhouse. The test plants were subjected to observation with a low-temperature cryofixation scanning electron microscope S-3400N (Cryo-SEM, Hitachi High-Tech, Tokyo, Japan). Observations were made for 5×5 mm pieces of cut leaf tissue placed on a specimen cooling stage and then transferred to the preparation chamber. Then, Cyro-SEM observations were performed −120°C at low voltage (5 kV)—using a secondary electron detector.
The antifungal activity of flutianil against different fungal species was investigated. No antifungal activity was observed in vitro against 24 investigated species of various pathogens (Table S1). Pot tests for the effects of flutianil on various pathogens indicated that flutianil did not show any efficacy—even at a high concentration of 500 mg/L—against B. cinerea, P. fulva, G. cingulata, M. nattrassii, P. infestans, and P. oryzae (Table 1). On the contrary, flutianil at 10 mg/L exhibited high activity (100% control) against powdery mildew on cucumber, eggplant, grape, and wheat. These results indicate that flutianil is effective only against powdery mildew on certain crops.
| Disease | Crop | Fungal species | Control valuec) | |||||
|---|---|---|---|---|---|---|---|---|
| 500a) | 200 | 10 | 1 | 0.1 | 0.01 | |||
| Powdery mildew | Cucumber | Podosphaera xanthii | 100 | 100 | 100 | 100 | 100 | 50 |
| Powdery mildew | Eggplant | Sphaerotheca fuliginea | NTb) | NT | 100 | 100 | NT | NT |
| Powdery mildew | Grape | Erysiphe necator | NT | NT | 100 | 100 | 100 | NT |
| Powdery mildew | Wheat | Blumeria graminis f. sp. tritici | 100 | 100 | 100 | NT | NT | NT |
| Powdery mildew | Barley | Blumeria graminis f. sp. hordei | 100 | 100 | 100 | NT | NT | NT |
| Gray mold | Cucumber | Botrytis cinerea | 0 | 0 | NT | NT | NT | NT |
| Leaf mold | Tomato | Passalora fulva | 0 | NT | NT | NT | NT | NT |
| Anthracnose | Strawberry | Glomerella cingulata | 0 | NT | NT | NT | NT | NT |
| Leaf mold | Eggplant | Mycovellosiella nattrassii | 0 | NT | NT | NT | NT | NT |
| Late blight | Tomato | Phytophthora infestans | 0 | NT | NT | NT | NT | NT |
| Blast | Rice | Pyricularia oryzae | 0 | 0 | NT | NT | NT | NT |
a) Concentration of flutianil (mg/L). b) NT: Not Tested. c) n=3.
The residual activity of flutianil was measured using flutianil-treated cucumber kept in a greenhouse for 14 days before inoculation. Flutianil at 10 mg/L exhibited 100% control against P. xanthii and the activity was maintained for at least 14 days. The rainfastness of flutianil was measured by placing the flutianil-treated cucumbers under artificial rainfall for either 3 or 24 hr. Flutianil at 10 mg/L maintained a high level of activity with both 3 and 24 hr of rainfall (control value: 100%). These results indicate that flutianil has excellent residual activity and rainfastness.
3. Translaminar and systemic activity of flutianilWhen flutianil was applied only on the adaxial or abaxial surfaces of cucumber leaves, it exhibited high control of pathogens existing on other leaf surfaces, control values being over 90% at 1 mg/L (Table 2). Thus, flutianil exhibits excellent translaminar activity. However, flutianil did not show any systemic activity since no control value was observed in inoculated cucumber leaves when flutianil was applied to the soil around cucumber plants. Flutianil did not show even local systemic activity, since only 40–50% control values were obtained when flutianil was applied either on the base or apex of the cucumber leaf after inoculating the entire leaf with P. xanthii (Table 2). These data indicate that flutianil used to treat cucumber plants moved from the leaf surface to the opposite leaf surface but was not translocated throughout the entire plant nor moved from base to apex or apex to base within the leaf.
| Concentration of Flutianil (mg/L) | Control valuef) | ||||
|---|---|---|---|---|---|
| Translaminar activity | Systemic activity | Local-systemic activity | |||
| Adaxial to abaxial a) | Abaxial to Adaxial b) | Soil to 1st stage of leaf c) | From base to apex d) | From apex to base e) | |
| 10 | 100 | 100 | 0 | 50 | 40 |
| 1 | 100 | 90 | 0 | 27 | 10 |
a), b) Flutianil was applied with a brush to the adaxial-leaf surface (a) or the abaxial (b) surfaces of the cucumber leaf 24 hr before inoculating P. xanthii. c) Flutianil was applied in the soil 24 hr before inoculating P. xanthii. d), e) Flutianil was applied on the base (d) or apex (e) of 1st stage of cucumber leaf 24 hr before inoculating P. xanthii. f) Control value was determined by observing the area of lesion of the 1st stage of leaves. n=3 plants per treatment.
Cross-resistance studies between flutianil and other existing (commercially available) fungicides were conducted using the isolates collected from cucumbers, strawberries, and grapes. The existing fungicides used in the studies were azoxystrobin (QoI, FRAC code 11), triflumizole (C14 demethylation inhibitor, FRAC code 3), a mixture of cyflufenamid (unknown mode of action, FRAC code U 06) and triflumizole, penthiopyrad (succinate dehydrogenase inhibitor, FRAC code 7), chinomethionat (multi-site contact activity, FRAC code M 10), and iminoctadine albesilate (multi-site contact activity, FRAC code M 07). Table 3 shows the efficacy of flutianil and four other fungicides against 12 isolates of P. xanthii collected from cucumbers in various locations of Japan. Flutianil (EC) completely controlled all isolates at concentrations of 1 and 10 mg/L, while, azoxystrobin, triflumizole, a mixture of cyflufenamid and triflumizole, and penthiopyrad were less effective against some P. xanthii isolates.
| Compound | Conc. (mg/L)a) | Control value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Labb) | STc)-1 | SG-1 | IB-1 | ST-3 | CB-1 | IB-4 | NI-1 | CB-5 | TK-1 | KU-3 | TK-2 | ||
| Jun-07 | Jul-07 | Aug-07 | Aug-07 | Jun-08 | Jul-08 | Jul-08 | Sep-08 | Oct-11 | Jun-12 | Apr-13 | Jul-13 | ||
| Flutianil 5EC | 10 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 3.3 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| 1 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| 0.3 | 70 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 83 | 50 | 100 | |
| 0.1 | 50 | 63 | 100 | 90 | 91 | 50 | 100 | 100 | 85 | 54 | 20 | 55 | |
| 0.03 | 30 | 50 | 71 | 60 | 13 | 38 | 0 | 50 | 55 | 17 | 0 | 0 | |
| Azoxystrobin 20SC | 133 | 10 | 25 | 48 | 70 | 6 | 0 | 3 | 20 | NTd) | 0 | 0 | 10 |
| 13.3 | 0 | 4 | 25 | 40 | 0 | 0 | 0 | 0 | NT | 0 | NT | NT | |
| Trifumizol 30WP | 100 | 100 | 91 | 94 | 30 | 19 | 56 | 90 | 100 | NT | NT | NT | NT |
| 10 | 10 | 29 | 0 | 20 | 0 | 0 | 15 | 45 | NT | NT | NT | NT | |
| Cyflufenamid+Triflumizole 3.4+15 WDG | 17+75 | 100 | 75 | 29 | 30 | 31 | 38 | 25 | 90 | 95 | 0 | 0 | 5 |
| 1.7+7.5 | 100 | 29 | 0 | 20 | 0 | 0 | 0 | 25 | 50 | 0 | NT | NT | |
| Penthiopyrad 20SC | 100 | 100 | NT | NT | NT | NT | NT | NT | NT | 98 | 42 | 10 | 25 |
| 10 | 75 | NT | NT | NT | NT | NT | NT | NT | 60 | 4 | NT | NT | |
a) Conc, concentration of test compounds. b) Lab, maintained in OAT Agrio’s laboratory. c) Sampling prefecture, abbreviations: ST, Saitama; SG, Saga; IB, Ibaraki; CB, Chiba; NI, Niigata; TK, Tokushima; KU, Kumamoto. d) NT: Not Tested.
Table 4 shows the efficacy of flutianil and six other fungicides against eight isolates of S. aphanis var. aphanis collected from strawberries in various locations of Japan. Flutianil (EC) completely controlled all 8 isolates at concentrations of 3.3 and 10 mg/L, while, azoxystrobin, chinomethionat, triflumizole, a mixture of cyflufenamid and triflumizole, iminoctadine albesilate, and penthiopyrad were less effective against some S. aphanis var. aphanis isolates. These findings indicate that there is no cross-resistance between flutianil and other existing fungicides for P. xanthii and S. aphanis var. aphanis.
| Compound | Conc. (mg/L)a) | Control value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lab-2b) | SMc)-1 | SG-2 | TC-7 | SZ-1 | TC-9 | TK-3 | KU-1 | ||
| Nsov-11 | Feb-09 | May-10 | May-10 | May-10 | Nov-11 | Nov-11 | May-12 | ||
| Flutianil 5EC | 10 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 3.3 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| 1 | 88 | 100 | 100 | 78 | 88 | 93 | 71 | 88 | |
| 0.3 | 58 | 70 | 88 | 83 | 67 | 61 | 46 | 88 | |
| 0.1 | 46 | 37 | 75 | 52 | 50 | 68 | 42 | 17 | |
| 0.03 | 50 | 23 | NTd) | NT | NT | 61 | 21 | NT | |
| Azoxystrobin 20SC | 133 | 100 | 80 | 100 | 78 | 54 | 100 | 100 | 71 |
| Chinomethionat 25WP | 83.3 | 19 | 53 | 100 | 87 | 42 | 82 | 8 | 8 |
| Triflumizole 30WP | 150 | 76 | 7 | 100 | 70 | 21 | 93 | 96 | 100 |
| Cyflufenamid+Triflumizole 3.4+15 WDG | 17+75 | 39 | 23 | 100 | 65 | 50 | 100 | 92 | 33 |
| Iminoctadine albesilate 30SC | 150 | 53 | NT | 100 | 78 | 83 | 93 | 46 | 25 |
| Penthiopyrad 20SC | 100 | 76 | NT | NT | NT | NT | 93 | 96 | 100 |
a) Conc, concentration of test compounds. b) Lab, maintained in the OAT Agrio’s laboratory. c) Sampling prefecture, abbreviations: SM, Shimane; SG, Saga; TC, Tochigi; SZ, Shizuoka; TK, Tokushima; KU, Kumamoto. d) Not tested.
Table 5 shows representative data for the EC50 values of flutianil and two other fungicides—quinoxyfen (signal transduction, FRAC code 13) and tebuconazole (C14 demethylation inhibitor, FRAC code 3)—against the E. necator isolate collected from grapes in various areas in Europe. The EC50 values for flutianil ranged from 0.01 to 0.18 mg/L, with the median value being 0.08 mg/L (some EC50 data not shown in the table were used for calculation). EC50 values for quinoxyfen ranged from 0.01 to >100 mg/L, with the median value being >100 mg/L, indicating considerable variation in the EC50 values among the isolates tested. The EC50 values for tebuconazole also varied widely, between 0.05 and 16.4 mg/L, with the median value being 5.71 mg/L. As shown in Fig. 2, there were no positive correlations between the EC50 values of flutianil and quinoxyfen (Fig. 2A) and/or tebuconazole (Fig. 2B). These data indicate that there was no cross-resistance between flutianil and quinoxyfen or tebuconazole for E. necator located in Europe.
| Strains | Country | Areas | Location | Date of sampling | EC50 values (mg/L) | ||
|---|---|---|---|---|---|---|---|
| flutianil | quinoxyfen | tebuconazole | |||||
| 5FBA01 | France | Languedoc | Boges | 22-May-05 | 0.02 | 0.08 | 0.05 |
| 7FBR03 | France | Armagnac | Bretagne d’Armagnac | 14-Aug-07 | 0.02 | 2.90 | 3.93 |
| 7PGR01 | Portugal | Oete | Gradil | 23-Jul-07 | 0.10 | 1.15 | 0.72 |
| 7IMT02 | Italy | Marche | Mont-prandone | 28-Aug-07 | 0.12 | 49.49 | 14.36 |
| 7FMB01 | France | Loire valley | Montreuil Bellay | 26-Sep-07 | 0.18 | 54.74 | 4.06 |
| 7GAL01 | Germany | Rhein-hessen | Alzey | 16-Oct-07 | 0.08 | 2.10 | 16.37 |
| 5FBR01 | France | Languedoc | Brouilla | 6-Oct-05 | 0.01 | 0.01 | NTa) |
| 8FBL04 | France | Loire valley | Bouille Lorets | 23-Jul-08 | 0.07 | >100 | NT |
| 8FMU07 | France | Burgundy | Meursault | 9-Sep-08 | 0.06 | >100 | NT |
| 8FSS02 | France | Loire valley | St. Martin de Sanzay | 23-Jul-08 | 0.17 | >100 | NT |
a) NT: Not Tested.
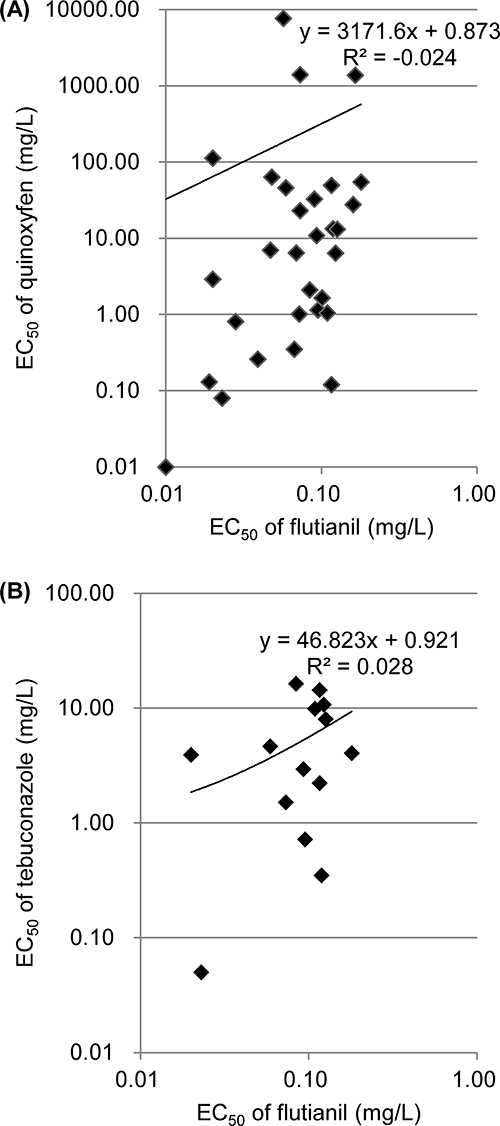
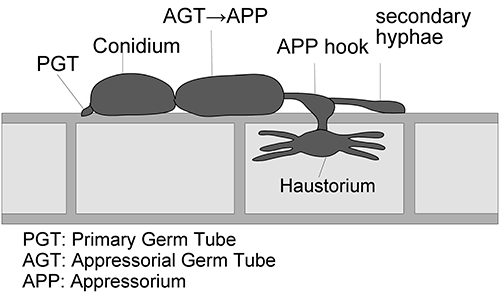
The effects of flutianil on the infection process of Bgh conidia were investigated using barley coleoptiles treated with 1 or 10 mg/L of flutianil and, at the same time, inoculated with Bgh conidia. Light microscopic observation 24 hr after the inoculation indicated that flutianil had almost no effect on conidial germination, PGT germination, AGT germination, APP formation, and APP hook formation in the early infection process of Bgh, but it significantly inhibited haustorium development (see Fig. 3 for infection process and morphological terminology). The rates of haustorium formation were only 3 and 9% by treatment with flutianil of 1 and 10 mg/L, respectively (Fig. 4). For comparison, 4 existing fungicides—cyflufenamid, benomyl, tolfenpyrad and triflumizole—were subjected to haustorium formation studies in the same manner as flutianil. The inhibitory rates of haustorium formation by cyflufenamid, benomyl, and tolfenpyrad, were the same as that of flutianil, but the inhibitory rate by triflumizole was significantly lower, as shown in Fig. 4.
Then, the inhibitory pattern of flutianil—inhibiting only haustorium formation but not conidium bursting, PGT germination, AGT germination, APP formation, and APP hook formation—was compared with those of 8 existing fungicides. As shown in Table 6, the inhibitory pattern of cyflufenamid was quite similar to that of flutianil since both fungicides inhibited only haustorium formation. Another 7 fungicides, such as triflumizole, azoxystrobin, quinoxyfen, and metrafenone, showed inhibitory patterns that were different from those of flutianil and cyflufenamid. For example, triflumizole inhibited APP formation and haustorium formation. Benomyl inhibited APP and APP hook formation as well as haustorium formation. Metrafenone inhibited both APP and APP hook formations.
| Compound | Conidium bursting | PGT germination | AGT germination | APP formation | APP hook formation | Haustorium formation |
|---|---|---|---|---|---|---|
| Flutianil | − | − | − | − | − | ++ |
| Triflumizole | − | − | − | ++ | − | + |
| Azoxystrobin | ++ | ++ | ||||
| Tolfenpyrad | − | − | + | ++ | ||
| Quinoxyfen | − | ++ | ++ | ++ | ++ | |
| Benomyl | − | − | − | ++ | ++ | ++ |
| Chinomethionat | − | ++ | ++ | ++ | ++ | |
| Cyflufenamid | − | − | − | − | − | ++ |
| Metrafenone | − | − | − | ++ | ++ |
a) +–++: Inhibited, −:not inhibited. Blank: Additional microscopic analysis of Bgh was unable to conduct because fungal development was not observed. PGT: Primary Germ Tube. AGT: Appressorial Germ Tube. APP: Appressorium.
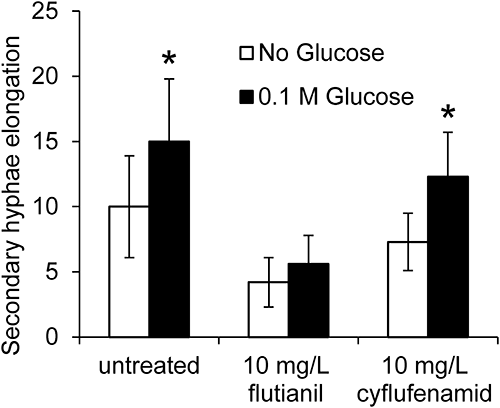
Since the inhibitory patterns of flutianil and cyflufenamid on Bgh conidial development were similar, they were investigated if there was any difference in secondary hyphal elongation when glucose was added as a nutrient to the Petri dish in which Bgh conidium–inoculated coleoptiles existed. The elongation rates of secondary hyphae for the untreated and cyflufenamid-treated specimens were 1.5 and 1.7 times higher, respectively, when 0.1 M glucose was added than when it was not added, as shown in Figs. 5 and 6. On the contrary, the elongation rate for flutianil-treated specimens was only 1.2 times higher, being significantly lower as compared to cyflufenamid-treated or untreated specimens. This indicates that the glucose absorption by haustoria and subsequent secondary hyphal elongation were significantly inhibited by flutianil but not by cyflufenamid, suggesting that flutianil’s mode of action is different from that of cyflufenamid.

The curative activity of flutianil was investigated by using inoculated cucumber leaves. It is well known that curative activity is observed when fungicides are applied after fungal infection but not before the onset of symptoms (pre-symptoms), while eradicant activity is observed when fungicides are applied after the onset of symptoms (post-symptoms).13) As shown in Table 7, when sprayed onto cucumber leaves 1 day after the inoculation of conidia, flutianil showed 100% control against P. xanthii. Flutianil’s inhibitory activity was maintained for up to 14 days after inoculation, although there was a gradual decrease in activity over the days following flutianil inoculation (50% inhibition on day 5 to 10% inhibition on day 14). Microscopic observation indicated abnormal conidiophore formation from 5 to 14 days (post-symptoms) after flutianil treatment, while no additional secondary P. xanthii infection was observed. Thus, flutianil demonstrated curative activity.
| Compound | Time interval between flutianil application and inoculation | ||||||
|---|---|---|---|---|---|---|---|
| Pre-symptoms | Post-symptoms | ||||||
| 1 da) | 5 d | 7 d | 9 d | 12 d | 14 d | ||
| 10 mg/L of flutianil | Control value | 100 | 50 | 25 | 30 | 20 | 10 |
| Sporulationb) | NF | ACF | ACF | ACF | ACF | ACF | |
| Secondary infectionc) | — | — | — | — | — | — | |
a) Flutianil was sprayed onto the cucumber leaves 1, 5, 7, 9, 12, and 14 days after inoculation, (n=3). b) NF, not formed; ACF, abnormal conidiophores formed. Observation was made 7 days after flutianil treatment by microscope, (n=3). c) —, not infected. (n=3).
Cryo-SEM observation was made to see the effect of flutianil on mycelium and conidiophore formation using flutianil-treated and untreated cucumber leaves 14 days after the inoculation of P. xanthii. The untreated leaves were found to be covered with mycelium and numerous conidiophores consisting of a mother cell that produced chains of conidia separated by regularly spaced septa (Fig. 7). On the contrary, in flutianil-treated (10 mg/L) leaves, conidiophores had formed abnormally as elongated tubes, but the collapse of conidiophores or mycelium was not observed.

It is interesting to note that flutianil has excellent antifungal activity against powdery mildew on various crops but no direct inhibitory effects on the growth of many other plant pathogenic fungi. High residual activity and rainfastness which were confirmed by current studies are characteristic features of flutianil. In addition, flutianil showed translaminar activity and curative activity at extremely low concentrations. Overall, these biological properties are likely to contribute to flutianil’s excellent efficacy in practical field conditions.
The inhibitory pattern of flutianil on powdery mildew is quite characteristic. Flutianil brought about significant reduction in the haustorium formation of Bgh conidia by preventive treatment and inhibited further fungal development up to the secondary hyphal elongation but did not inhibit conidium bursting, PGT and AGT germination, and APP and APP hook formations. By curative treatments, flutianil also inhibited the late infection processes of the pathogen, including the formation of conidiophores and the release of conidia from conidiophores. This inhibitory pattern of flutianil in the infection process is quite different from those of other existing fungicides. For example, triflumizole has been shown to affect the formation of APP hook and haustorium.14) Azoxystrobin has been shown to significantly reduce the spore germination of Cladosporium and Mycosphaerella.15) Quinoxyfen is reported to significantly inhibit the formation of APP and APP hooks.16) Metrafenone has been shown to inhibit the germination of conidia and to affect the germination location; subsequently formed APP have frequently presented two or three lobes, which have often been malformed.17) Cyflufenamid it inhibits the formation of haustoria as does flutianil, but it has been shown not to affect the germination of conidia or the formation of APP.18)
The inhibitory pattern of flutianil in the life cycle of powdery mildew is thus quite different from that of existing fungicides except for cyflufenamid. Flutianil and cyflufenamid showed the same inhibitory pattern up to the haustorium formation stage in the life cycle of powdery mildew. However, there were differences between the two fungicides in the nutrient absorption by haustoria and subsequent secondary hyphal elongation, which was inhibited by flutianil but not by cyflufenamid. More evidence of the different inhibitory patterns is flutianil’s effectiveness against P. xanthii and S. aphanis var. aphanis, against which cyflufenamid is less effective. It is assumed that flutianil’s mode of action is different not only from that of cyflufenamid but also from those of other existing fungicides.
It is interesting to know whether the action of flutianil, an antifungal agent, is fungistatic or fungicidal. In general, fungistatic compounds are able to slow or retard the multiplication of fungi, as represented by fluconazole, which inhibits the growth of fungi.19) In contrast, fungicidal compounds, such as azoxystrobin, kill the fungal pathogens in action.20) Since powdery mildew fungus is an obligate biotroph, it is not possible to directly judge whether flutianil kills the fungus. Current curative treatment studies for flutianil, as well as morphological studies of Bgh and P. xanthii, have revealed that flutianil caused the formation of abnormal conidiophores, like elongated tubes. However, the collapse of the conidiophores or mycelium was not observed, and the pathogen still seemed alive. Thus, flutianil has fungistatic rather than fungicidal properties. It has been reported that the difference between fungicidal and fungistatic action somehow depends on the concentration of the antifungal agents.21) In the case of flutianil, the optimal concentration to express antifungal effects in pot tests was less than 10 mg/L. Even at 100 mg/L, flutianil did not cause the collapse of the fungal body, although the data are not shown in the text. The action of flutianil still seems to be fungistatic rather than fungicidal on Bgh and P. xanthii. Further studies on the fungistatic properties of flutianil are now in progress.
Current studies indicate that flutianil has curative activity against powdery mildew in addition to preventive activity, since flutianil treatment on cucumber leaves 5–14 days after the inoculation of P. xanthii inhibited the secondary infection of conidiophores and conidial release through morphogenic effects. Flutianil can be used preventively or in a very early stage of disease infection to achieve optimal control. The use of flutianil in this way is especially important to prevent or delay the development of resistance. A curative treatment with flutianil in the post-symptom stage may increase the risk of resistance development due to an increase of the allowed frequency of isolate survival, which would consequently accelerate the speed of resistant isolate selection.
When launching new fungicides, the establishment of baseline sensitivity data for them is very important from the perspective of managing fungicide resistance. Baseline sensitivity data can be used to illustrate shifts in pathogen sensitivity and provide evidence that any control failures might be correlated with the appearance of resistance in the population.22) It is useful that the baseline sensitivity data for E. necator with flutianil was established by determining the control values of flutianil in various areas in Europe by leaf disc assay.
As mentioned, flutianil exhibited excellent control against P. xanthii at a very low concentration, and it possibly acts on specific target sites of powdery mildew. Detailed studies on the mode of action of flutianil are in progress.
The authors gratefully acknowledge the work of past and present members of Research and Development Division of OAT Agrio Co., Ltd. We also wish to thank Dr. N. Umetsu, Visiting Professor of Kibi International University for helpful discussion and comments on the manuscript.
The online version of this article contains supplementary material (Supplemental Table S1), which is available at https://www.jstage.jst.go.jp/browse/jpestics/